All published articles of this journal are available on ScienceDirect.
A Comprehensive Review of COVID-19 Transmission and Vaccine Development: Past, Present, and Future Prospects
Abstract
Corona virusis a large group of viruses that cause respiratory and gastrointestinal illnesses. Originating in Wuhan, China, in December 2019, the 2019-novel Corona virus pandemic has spread around the globe and raised concerns. Due to the large number of individuals affected worldwide, the illness has rendered isolated areas uninhabitable, forcing residents to stay inside their homes in an effort to contain its spread. The 2019 corona virus, the severe acute respiratory syndrome corona virus, and the first human pandemic of the twenty-first century have identical human cellular receptors. Nevertheless, compared to the severe acute respiratory syndrome corona virus, the 2019-novel corona virus is more powerful, highly infectious, and changeable. The spike glycoprotein is the best place to create a 2019 corona virus vaccine. Where would be best to develop a vaccine against the 2019 novel Numerous mechanisms, including receptor binding, membrane fusion via conformational changes, viral internalization, host tissue tropism, and spike deactivation due to antibody-induced instability, depend on the spike glycoprotein known as corona virus. After the first breakout in December 2019, everyone in the world felt momentarily comforted when the death ratio began to decline around the end of 2020. People believed that the summer was one of the best seasons to combat illness and prevent its spread. However, in recent months, a global outcry over new 2019 Corona virus infection variations has garnered attention, putting people's lives, regardless of age or community, at risk. Scholars must concentrate on the findings and advancements. In addition, we have worked to increase awareness of the need for the creation of an international virtual community in order to enable smooth communication across all parts of the world and support mankind in the case of a category 5 coronavirus outbreak.
1. INTRODUCTION
In December 2019, there was an unknown cause pneumonia epidemic in Wuhan, in the Chinese province of Hubei. A few days later, many different labs determined that a new corona virus, or nCoV, was the main cause of this unexplained pneumonia [1-4]. The World Health Organization has temporarily identified the causative virus as severe acute respiratory syndrome corona virus 2 (SARS-CoV-2). The corona viruses (CoVs) are a very diverse family of enclosed, positive-sense, single-stranded RNA viruses [5]. They cause a variety of diseases that impact the neurological, hepatic, pulmonary, and gastrointestinal systems to different degrees in both people and animals [5, 6]. Symptomatic therapy is the mainstay of care for COVID-19 patients. Using antiviral drugs like lopinavir, ritonavir, ganciclovir, ribavirin, and oseltamivir in many studies has been done to try to lower the viral load and lower the risk of respiratory problems [7-11].
Much research has gone into developing COVID-19 vaccines in an attempt to avert the pandemic, and most of these candidates have made use of the S-protein of SARSCoV-2 [12]. There were 158 potential SARSCoV-2 vaccine candidates worldwide as of 2020, 135 of whom were in the preclinical or exploratory phases of research. Vaccinations in the channel must use viral vectors that can copy themselves as well as those that can't. They also require protein subunits, DNA, RNA, nanoparticles, live or inactive attenuated viruses, and viral vectors [13]. Hubei. A few days later, many different labs determined that a new corona virus, or nCoV, was the main cause of this unexplained pneumonia [2, 4]. The World Health Organisation has temporarily identified the causative virus as severe acute respiratory syndrome corona virus 2 (SARS-CoV-2). The corona viruses (CoVs) are a very diverse family of enclosed, positive-sense, single-stranded RNA viruses [5]. They cause a variety of diseases that impact the neurological, hepatic, pulmonary, and gastrointestinal systems to different degrees in both people and animals [5, 6]. Symptomatic therapy is the mainstay of care for COVID-19 patients. Using antiviral drugs like lopinavir, ritonavir, ganciclovir, ribavirin, and oseltamivir in many studies has been done to try to lower the viral load and lower the risk of respiratory problems [7-11]. Much research has gone into developing COVID-19 vaccines in an attempt to avert the pandemic, and most of these candidates have made use of the S-protein of SARSCoV-2 [12]. There were 158 potential SARSCoV-2 vaccine candidates worldwide as of 2020, 135 of whom were in the preclinical or exploratory phases of research. Vaccinations in the channel must use viral vectors that can copy themselves as well as those that can't. They also require protein subunits, DNA, RNA, nanoparticles, live or inactive attenuated viruses, and viral vectors [13].
With 44,998,525 cases and 532,031 fatalities as of September 23, 2023, India is the nation with the third-highest number of COVID-19 cases globally, after only the United States and China [14]. On January 27, 2020, the evacuation of an Indian person from China led to the first COVID-19 case, and since then, the number of cases has been steadily increasing [15]. Three individuals who had visited China confirmed the condition on February 3, 2020, leading to the country's first report of the ailment [16]. No new cases were reported in February, but by mid-March, several cases had been reported, with sporadic reports coming from all states in India [17]. By April, the illness had affected all states except Sikkim. In March 2020, India reported the first COVID-19 fatality [18]. Maharashtra (81,71,453), Kerala (69,07,284), Karnataka (40,88,819), Tamil Nadu (36,10,676), Andhra Pradesh (23,40,676), Uttar Pradesh (21,45,443), West Bengal (21,26,370), and Delhi (20,40,949) were the states with the highest number of cases recorded during the epidemic [19]. The whole country was under lockdown from March to May, which helped to lower the peak number of cases [20]. The lockdown shortened the doubling of incidents from three days to six days. We implemented preventive and public health measures, such as quarantining positive patients for 14 days and isolating suspicious cases, to slow down the spread. the illness from spreading, preventive measures have been put in place, such as checking immigrants at airports [21]. As of September 24, 2023, there were 44,998,525 total instances, of which 469 were active. As of September 24, 2023, hospitals discharged 44,466,025 patients, and 5,32,031 people died nationwide a CFR of 1.18% [19]. The primary forms of Covid-19 epidemiology were animal-to-human and human-to-human transmission in the beginning.
Many accounts support the idea that humans contract SARS-CoV-2 from animals, even though the precise source of the virus remains unclear [22]. Both SARS-CoV-2 and SARS-CoV may spread directly from person to person because they have comparable genomic and phylogenetic features, as well as identical receptor-binding domains and spike gene sequences [23]. Following that, people with symptomatic COVID-19 pneumonia are among the primary causes believed to be responsible for SARS-CoV-2 propagation from person to person. Moreover, it remains possible that those who are known as “superspreaders” have a major impact on the rapid spread of the pandemic virus [24]. Researchers have proposed that respiratory droplets and close contact between individuals are the main ways that SARS-CoV-2 spreads [25]. However, there is mounting evidence that the virus may spread via aerosols and fumes and that it can remain infectious on surfaces for many days and hours in aerosols [26].
2. VACCINE AND ITS PROGRAMS
Covishield, a product of the British-Swedish pharmaceutical giant AstraZeneca and the University of Oxford, boasts an efficacy of 90 to 95 percent when administered two to three months apart. The Serum Institute of India (SII), which is also producing the Covishield vaccine for the mass immunization campaign, carried out the experiment in India. The Serum Institute of India (SII) created the Covishield vaccination using the adenovirus that causes chimpanzees to get the common cold. Its genetic makeup is identical to that of the SARS-CoV-2 corona virus’s spike protein. Spike protein is the portion of SARS-CoV-2 that allows the virus to enter a human body cell [27-29].
The main parts of the Covishield vaccine are L-histidine hydrochloride monohydrate, polysorbate 80, ethanol, aluminium hydroxide gel, magnesium chloride hexahydrate, sodium chloride, EDTA (also called disodium edetate), and an adenovirus that has been disabled and modified to contain specific coronavirus segments [30]. An intramuscular injection administers the vaccination. The covishield vaccine is essentially a replication-lagging simian adenovirus vector that contains a plasminogen activator (tPA) leader sequence and the whole codon-optimized coding sequence of SARS-CoV-2's S protein [31, 32]. The S protein of the SARS-CoV-2 virus does not assist the adenovirus in replicating because it replaces several essential genes with those in the adenovirus [33, 34]. After vaccination, the body produces the S protein, preparing its immune system to combat the SARS-CoV-2 virus should it subsequently strike. Keep the Covishield vaccination refrigerated between 2°C and 8°C for optimal results [35, 36]. There is a 4-to 12-week interval between the two doses of the Covishield vaccination [37]. Upon immunization, the adenovirus vector enters the cells. The adenovirus vector then releases its genetic material and transmits it to the cell nucleus. Subsequently, the cell proceeds through transcription, producing mRNA and proteins (via translation). The SARS-CoV-2 virus starts when an outside protein enters the cell through the ACE2 (angiotensin-converting enzyme 2) enzymatic domain. The S protein is the protein of interest [38-40]. The vaccine primes the body to fight the SARS-CoV-2 virus in the future by using T cells, also known as T lymphocyte cells and antibodies [41].
3. GLOBAL VACCINATION PROGRAM
The World Health Organization has served as a hub for the integration and exchange of research data and findings. In addition to launching creative projects to hasten the development of treatments and vaccinations via international cooperation, WHO has established criteria for country governments to follow when investigating and tracking down COVID-19 cases [42]. In addition to WHO, a number of specialist international research collaborations are essential to the coordination of worldwide activities. These include the following: The Wellcome Trust, the Bill & The EU Commission, and consequently, the European Federation of Pharmaceutical Industries and Associations collaborated on the Innovative Medicines Initiative (IMI), a public-private collaboration, to expedite emergency medical treatments. Philanthropies, pharmaceutical companies, and national governments form the Global Alliance for Vaccinations and Immunisation (GAVI), a public-private collaboration that aims to ensure equitable access to vaccinations for children in impoverished nations. Melinda Gates Foundation and a group of governments co-founded the Coalition for Epidemic Preparedness Innovations (CEPI), a global coalition to fund and organize the creation of vaccines for emerging infectious diseases. The Global Research Collaboration for Communicable Disease Preparedness, a network of international research funding organizations, facilitates research on infectious illnesses with pandemic potential. A global federation of clinical research networks, the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) aims to provide a coordinated research response to infectious illnesses that are prone to outbreaks [43-47].
4. COVID-19 EPIDEMIOLOGY
The medium-sized RNA viruses, which have a diameter of 65–125 nm and a genomic size ranging from 26 to 32 kilo bases, grow in the cytoplasm of human-infected cells. Human amino peptidase-N binds to group I corona virus (229E), 9-o-acetylated neuraminic acid binds to strain agriculture 43 (OC43), HCoV-HKU1, dipeptidyl-peptidase-4 binds to MERS-CoV, and agnomens [48-51]
SARS-CoV-2 has infected 120,000 individuals in 109 countries in the short period since it first surfaced in December 2019; the disease has a 2.9% mortality rate. The N501T mutation in the spike glycoprotein makes the identical ACE2 (angiotensin-converting enzyme) SARS-CoV2 more likely to bind to cell receptors [48]. The corona virus spike (S) proteins are the most interesting target for inhibitors and vaccines. Each spike monomer is made up of S1 and S2 subunits, which give the virus protein a crown-like shape that depends on sunlight while being positioned perpendicular to the cell membrane [52, 53]. We identified the arrangement of SARS-CoV's hydrophobic and aromatic domains in relation to one another as the fundamental element affecting its capacity to bind to receptors or fuse membranes [54].
HCoVs may use endosomal or non-endosomal routes, or sometimes a mix of both, to enter host cells. A conformational change in the S protein, resulting from the interaction between the S1 domain and its matching receptor via the S2 domain, facilitates membrane fusion between the viral and cell membranes. Specific factors such as low pH in the cellular milieu, specific proteases like cathepsin-L and transmembrane protease serine 2, and proteases mimicking trypsin in the airway stimulate these pathways [51]. Intestinal mucosal cells, renal tubular cells, immune cells, and neuronal dendritic cells all experience apoptosis as a result of HCoVs, which suppress the host's innate and adaptive immune responses [51]. SARS-CoV was able to cross species barriers and propagate from person to person because of its high contagiousness [51, 55]. Respiratory secretions, aerosols, faeces, or urine droplets can transmit it, keeping it infectious for seven days [56].
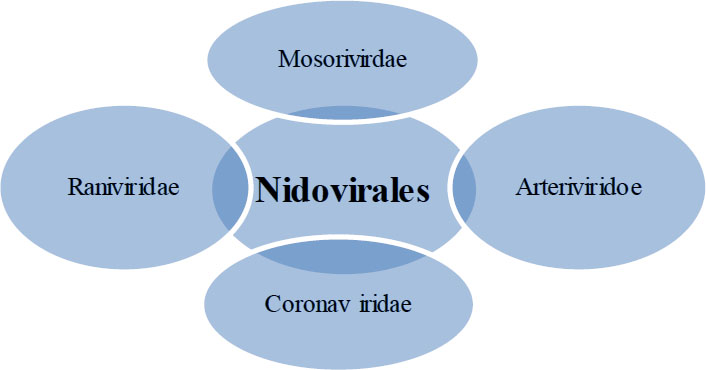
RNA-recombination in S-glycoprotein among the existing coronaviruses produced the formation of new coronaviruses, including 2019-nCoV from SARS-CoV, which had an outbreak as a devastating pandemic spreading from Wuhan, Hubei province of China, to almost every area of the globe. We subsequently dubbed it SARS-CoV-2 [57-59]. The genes for RNA-dependent RNA polymerase (Nsp12), S-glycoprotein, and proteases (such as 3-C and papain-like proteases) are the same in both SARS-CoV and SARS-CoV-2 [60]. Phylogeny and serology have separated HCoV into four genera. Please refer to Fig. (1) to see an example of the HCoV classification tree. Table 1 outlines the history and pandemic details of several HCoV strains [61].
It's possible that homologous recombination at the S-glycoprotein of the receptor binding domain (RBD) region makes it easier for the virus to spread between species. This region is believed to be crucial for receptor binding membrane fusion via conformational changes, viral internalization, host tissue tropism, or antibody-induced instability leading to spike deactivation [48, 50, 57-60]. Jian says that the SARS-CoV-2 RBD is better at recognizing ACE2 receptors than the SARS-CoV RBD because the SARS-CoV-2 receptor binding motif has developed ways to neutralize the charges on the lysine residues, which is a key feature for coronavirus RBD to bind to ACE2 [50, 53, 60]. COVID-19 attaches itself to ACE2, predominantly expressed on the surface of lung alveolar cells. Men and older adults had higher plasma concentrations of circulating ACE2, which raises the risk of infection for those with underlying medical conditions, healthcare professionals, and the elderly [62, 63].
Given the close relationship between 2019-CoV and SARS-CoV, the suggestion is to develop a peptide-based vaccination to prevent infection. Researchers have identified several distinct cytotoxic T-lymphocyte epitopes and vectors on SARS-CoV-2 [57, 64, 65]. Remdesivir blocks the coronavirus's RNA-dependent RNA polymerase, which is necessary for the transcription of its genes and genome replication. This led to its initial approval for emergency treatment of SARS-CoV-2 [57, 66]. Studies have shown that intravenous gamma globulins, Chinese remedies, and immune boosters may help reduce coronavirus symptoms.
Labs around the world are conducting several investigations to identify medicinal plants containing anti-viral phytochemicals. This could potentially halt a viral onslaught, prevent the virus from spreading, and aid in the development of novel COVID-19 vaccines and treatments [67, 68]. China used a diagnostic tool, lactic dehydrogenase, to identify high-risk COVID-19 patients and create an interpretable mortality prediction model [57, 69]. Certain cases used Dexamethasone, a glucocorticoid, to reduce COVID-19 patient mortality by one-third [70]. People have long used this inexpensive drug as an anti-inflammatory and anti-cancer treatment. A few studies, part of collaborative research across many universities, have used convalescent plasma treatment to boost COVID-19 patients' immune systems, enabling them to fight the virus with antibodies [71].
Classification of human coronaviruses. HCoV, human coronavirus.
| Serial No. | Human Coronavirus | Year of Outcome |
|---|---|---|
| 1 | B814 | 1965 |
| 2 | HCoV-229E | 1966 |
| 3 | Human coronavirus for organ culture-43 | 1967 |
| 4 | Severe acute respiratory syndrome coronavirus | 2003 |
| 5 | HCoV-NL63 | 2004 |
| 6 | HCoV-HKU-1 | 2005 |
| 7 | Middle East respiratory syndrome coronavirus | 2012 |
| 8 | Severe acute respiratory syndrome coronavirus-2 (coronavirus disease 2019) | 2019 |
5. VACCINE TYPE & MODE OF ACTION
Vaccines against entire pathogens, which are the most often used kind, typically include complete organisms that have been either killed or made less dangerous in order to prevent them from causing sickness. However, a secondary infection elicits a strong immune response. These are some of the first immunizations, developed in the late 1800s. Research indicates that the development of the first immunizations occurred in the late 1800s. Studies have demonstrated the ab.
The type of vaccine and the method of action are important considerations. Vaccines against entire pathogens, which are the most often used kind, typically include complete organisms that have been either killed or made less dangerous in order to prevent them from causing sickness. However, a secondary infection elicits a strong immune response. These are some of the first immunizations, developed in the late 1800s. Studies have demonstrated that inactivated microorganisms have the ability to boost immunity, leading to the development of inactivated vaccines. During the manufacturing process of these vaccinations, a variety of physical and chemical methods destroy the pathogen [72]. Vaccines against entire pathogens, which are the most often used kind, typically include complete organisms that have been either killed or made less dangerous in order to prevent them from causing sickness. However, a secondary infection triggers a strong immune response. These are some of the first immunizations, developed in the late 1800s. Studies have demonstrated that inactivated microorganisms have the ability to boost immunity, leading to the development of inactivated vaccines. These immunizations use a range of chemical and physical techniques to completely remove the virus [73]. Betapropiolactone treatment often stops the flu virus from working while the inactivated flu (flu) vaccine is being made (Kon et al., 2016). Beta-propiolactone treatment often stops the flu virus from working while the inactivated flu (flu) vaccine is being made (Kon et al., 2016) [74].
Tissue engineering advancements in the 1950s allowed researchers to make live-attenuated vaccinations. These vaccinations, which originate in vivo, include live germs that have lost some of their pathogenicity and have the ability to provide lifetime protection after a few doses (typically one or two). These immunizations often target viruses because bacteria, with their hundreds of genes, are more complex to manufacture than viruses [75]. Attenuation is the effect of growing the pathogen in animals or artificial host cell lines. Additionally, we can create attenuated vaccines using naturally existing, non-pathogenic strains of the same organisms. Tissue engineering has proven to be yet another crucial step in the attenuation process. For example, the transmission of the virus via animals, cell lines, or embryonated eggs reduces the effectiveness of the Rabies vaccination. Because we can choose genetically homogenous virus strains [76], we use modern methods. Lately, increasing polymerase fidelity for stable attenuation is another common use of reverse genetics. For example, RNA-dependent RNA polymerase activity in a poliovirus strain (Vignuzzi, Wendt, & Andino, 2008) [77, 78] is one example. Another is changing the activity of enzymes or proteins needed for virus replication, like methyltransferase activity in Avian metapneumovirus (Sun, Gardner, Watson, Ryman KD, & Klimstra, 2014) [77, 78]. A few other examples include the vaccinations against cowpox, BCG, and MMR. Complicated culture, attenuation management, genetic stability maintenance, and processing issues downstream have hindered the development and production of these vaccines. Inactivated microorganisms have the ability to boost immunity, which has led to the development of inactivated vaccines. During the manufacturing process of these vaccinations, a variety of physical and chemical methods destroy the pathogen [72]. Vaccines against entire pathogens, which are the most often used kind, typically include complete organisms that have been either killed or made less dangerous in order to prevent them from causing sickness. However, a secondary infection triggers a strong immune response. These are some of the first immunisations, developed in the late 1800s. Studies have demonstrated that inactivated microorganisms have the ability to boost immunity, leading to the development of inactivated vaccines. These immunizations use a range of chemical and physical techniques to completely remove the virus [74]. Betapropiolactone treatment often stops the flu virus from working while the inactivated flu (flu) vaccine is being made (Kon et al., 2016). Beta-propiolactone treatment often stops the flu virus from working while the inactivated flu (flu) vaccine is being made ([74] Tissue engineering advancements in the 1950s allowed researchers to make live-attenuated vaccinations. These vaccinations, which originate in vivo, include live germs that have lost some of their pathogen city and have the ability to provide lifetime protection after a few doses (typically one or two). These immunizations often target viruses because bacteria, with their hundreds of genes, are more complex to manufacture than viruses [75]. Attenuation is the effect of growing the pathogen in animals or artificial host cell lines. Additionally, we can create attenuated vaccines using naturally existing, non-pathogenic strains of the same organisms. Tissue engineering has proven to be yet another crucial step in the attenuation process. For example, the transmission of the virus via animals, cell lines, or embryonated eggs reduces the effectiveness of the Rabies vaccination. Because we can choose genetically homogenous virus strains [76], we use modern methods. Lately, Increasing polymerase fidelity for stable attenuation is another common use of reverse genetics. For example, RNA-dependent RNA polymerase activity in a poliovirus strain (Vignuzzi, Wendt, & Andino, 2008) [77, 78] is one example. Another is changing the activity of enzymes or proteins needed for virus replication, like methyltransferase activity in Avian metapneumovirus (Sun, Gardner, Watson, Ryman KD, & Klimstra, 2014) [77, 78]. A few other examples include the vaccinations against cowpox, BCG, and MMR. Complicated culture, attenuation management, genetic stability maintenance, and processing issues downstream have hindered the development and production of these vaccines.
6. SHOTS TO PREVENT INACTIVATED VIRUSES
Beijing Institute of Biological Products and Sinovac, a Chinese business, have received FDA approval for their inactivated viral vaccines, which include the SARS-CoV-2 vaccine.
7. NIDOVIRALES
7.1. Using Next-generation Technologies for Screening
Immunizations were created using a fairly basic technique, mixing attenuated and inactivated viruses, as immunology and vaccine production grew. However, over the last thirty years, advances in technology and computing have led to the development of highly immunogenic recombinant protein antigens, as well as significant progress in the fields of drug discovery and computational biology [79-81]. Reverse vaccinology, or RV, evolved when the scientific community attempted to unravel the riddles surrounding genetic sciences. Without having to grow the microorganism in the lab, RV uses computer methods to conceptually build vaccines using information found in the genetic sequences of pathogens [11]. For this purpose, CTLPred, NetCTL, Epitopemap, PickPocket, GPS-MBA, FRED2, MHC2 Pred, and ProPred-I software are now available. As an alternative, the algorithm must recognize antigens that can recognize B cells in situations where a humoral immune response is required, such as with viruses like HIV [12, 82].
8. COVID-19 HAS THE POTENTIAL TO IMPACT BOTH THE WORLD AND INDIA
The scientific community has united to create medications and treatments in response to the worldwide COVID-19 pandemic, as well as to uncover a potential vaccine candidate. Science began to move at an almost unnatural speed as soon as China released the genome of the new coronavirus, the source of the Wuhan outbreak. To create the quickest vaccination, researchers are now using machine learning, structural and computational biology, and genomics. As soon as ten weeks after the public release of the SARS-COV2 gene sequence, several potential vaccine candidates began Phase I clinical studies.
As of January 15, 2021, 64 vaccines were in clinical development, and 173 were in pre-clinical development globally. More than fifteen vaccines, including the innovative m-RNA vaccine from Moderna, Inc., are currently undergoing phase 3 clinical studies for initial use. Sputnik-V, developed by the Gamaleya Russian National Research, is the first approved COVID-19 vaccination. Pfizer, Moderna, and Bharat Biotech, among others, subsequently created and studied numerous additional vaccines. Moderna Inc. created the first mRNA vaccination in history to receive FDA approval. In January 2021, Sum Institute of India granted permission to the Indian Council of Medical Research to use the Oxford University-developed Covid Shield vaccine.
CONCLUSION
The benefits and drawbacks of vaccine administration, as well as vaccination epidemiology should be of interest to both the general population and academics. This knowledge has the potential to save many more lives by educating individuals about diseases that vaccination may prevent. By researching the past, scientists can develop vaccines for the future. Under the global COVID-19 framework, more than ten vaccines have received licenses fewer than 15 months after the outbreak started. Policymakers and academic organizations have worked together to make this possible. With the advent of new technologies, vaccine research, and bioproduction might see a revolution. Investing additional time and energy in computational design and prediction may save resources. A lot of progress has been made in finding new drugs and using computers to study biology. Researchers have made significant progress in finding new drugs and using computers to study biology. This, along with the ability to make highly immunogenic recombinant protein antigens thanks to advances in technology and computers, has made modern vaccination strategies possible. The possibility of developing next-generation vaccinations that may provide protection against any infection has been made possible by these advancements. In order to give readers a thorough understanding and motivate them to be ready for future disease outbreaks, the study concludes by summarizing the crucial components of vaccine development, approval, and design.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| SARS-CoV-2 | = Severe Acute Respiratory Syndrome Corona Virus 2 |
| CoVs | = Corona Viruses |
| SII | = Serum Institute of India |
| ACE2 | = angiotensin-converting enzyme 2 |
| ISARIC | = International Severe Acute Respiratory and Emerging Infection Consortium |
| RBD | = Receptor Binding Domain |
