All published articles of this journal are available on ScienceDirect.
Recent Trends of Therapeutic Strategies against COVID-19
Abstract
Background:
The coronavirus disease 2019 (COVID-19), caused by a virus named SARS-CoV-2, has spread rapidly around the world.
Objective:
To better understand the recent development of therapeutic strategies against COVID-19 and the patent landscape, we analyze patent documents surrounding COVID-19.
Methods:
The patent documents surrounding COVID-19 from 6 major countries, including China, the US, Russia, Korea, India, and Singapore, were analyzed by a comprehensive analysis. The patent documents surrounding COVID-19 were published from November 2019 to April 2021.
Results:
The analysis showed that China was the most prolific country in patents surrounding COVID-19, with over 300 published patent documents. A significant feature of therapeutic strategies against COVID-19 was the contribution of traditional Chinese medicines in China. Our study showed the number of patents in the therapeutic area was about half in all patent applications, which indicated that therapeutic approaches, detecting, and protecting approaches were the same in importance against COVID-19.
Conclusion:
The main therapeutic strategies against COVID-19 include traditional Chinese medicines, chemical drugs, and vaccines. An effective and fast approach against COVID-19 is to use vaccines or traditional Chinese Medicines. The present study showed the development of a therapeutic strategy surrounding COVID-19 based on patent insight for the first time and provided new insight into therapeutic strategy against COVID-19.
1. INTRODUCTION
Coronavirus disease 2019 (COVID-19) has rapidly spread around the world. COVID-19 has become a pandemic [1], and has been becoming one of the most significant challenges humankind has faced since World War Two [2, 3]. The emergence of COVID-19 has impacted people worldwide tremendously. So far, globally, about 254 million people have been confirmed with infections of COVID-19, and about 5 million people have died [4]. It is more serious in some developing countries, e.g., there were about 352 thousand confirmed cases of COVID-19 and over two thousand deaths in India on 26 April 2021 [5]. COVID-19 is caused by SARS-CoV-2, and its transmission rate is even higher than SARS-CoV [6-10]. The people with infection of COVID-19 are about 6-17% mortality rate for people [6, 11]. At the early stage, people who are infected with COVID-19 are asymptomatic infection but then manifest to be an acute respiratory infection [12, 13]. Eventually, pneumonia occurs, and bone marrow cells can be infected [14].
Many therapeutic strategies were being developed against COVID-19. To better understand the development of therapeutic strategy and patent landscape about COVID-19, we analyzed patent documents surrounding COVID-19 from 6 major countries, including China, the US, Russia, Korea, India, and Singapore, with the patents published from November 2019 to April 2021. We used the keyword TA “COVID-19” to identify relevant documents. PatSnap was used to search and analyze the data. This article provided the current patent landscape surrounding COVID-19 from major countries, including China, the US, Russia, Korea, India, and Singapore.
2. MATERIALS AND METHODS
2.1. Patent Filing Profile
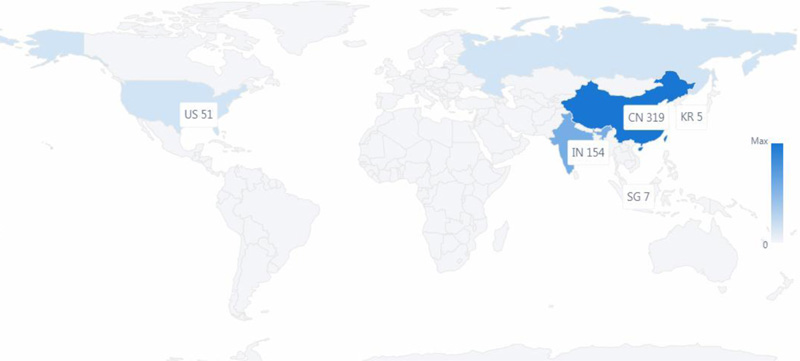
There were 559 published patent documents from the six countries, as shown in Figs. (1 and 2). The first granted patent in China was in June 2020. The time from the patent application to be granted was about three months. The first granted patent in Russia was in June 2020, the first granted patent in the United States was in Sept 2020, and the time from the application to be granted was about four months. These are the two fastest countries in completing grants of the patents.
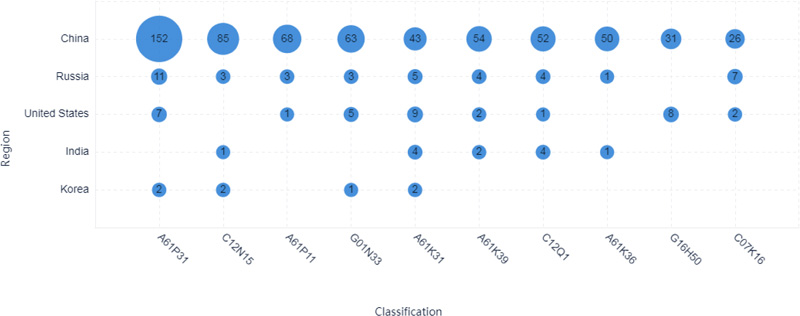
In terms of patent filing numbers, as shown in Fig. (2), China was a leading applicant for patents surrounding COVID-19, which filed over 300 patent applications. China made an enormous contribution to patent technologies surrounding COVID-19. India appears to be the second strongest applicant surrounding COVID-19, as shown in Fig. (2), the number of published patent documents exceeds the United States. The United States is the third in the number of published patent documents, which is about 1/2 of India in the number of patents.
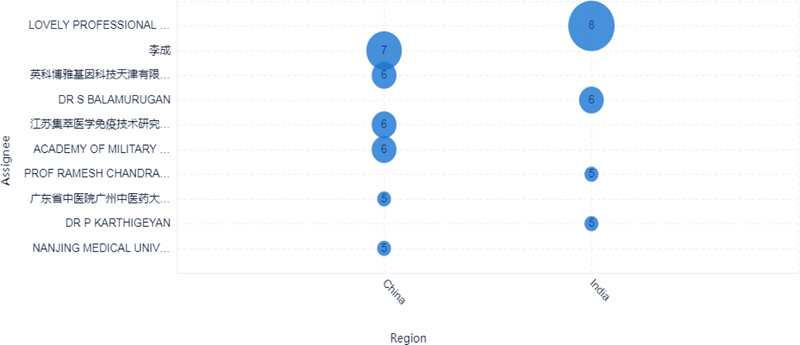
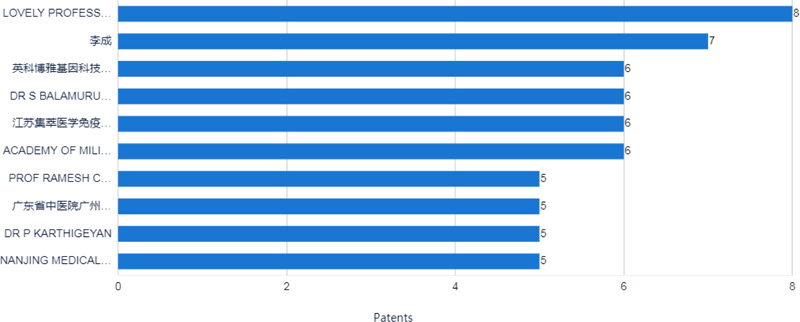
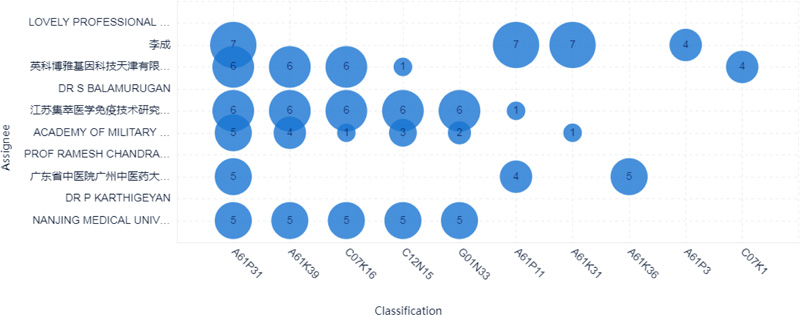
The top 10 applicants for patents surrounding COVID-19 were mainly from China and India, as shown in Fig. (3). Six applicants were from China, and the other four applicants were from India. Lovely professional university from India is the most prolific assignee with eight documents. The second prolific assignee is Li Cheng from China, with seven documents, as shown in Figs. (3-4).

2.2. Technology Landscape
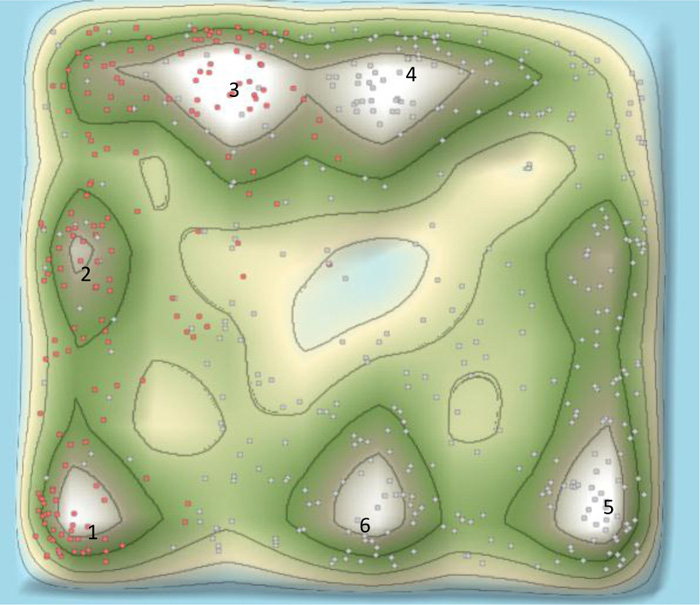
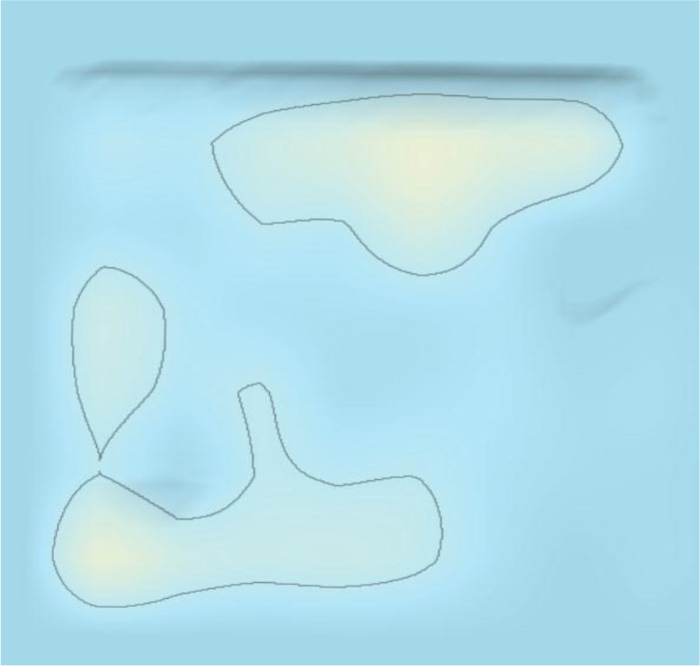
The 3D landscape of the patent surrounding COVID-19 provides a 3D macroscopic overview of patenting technology clusters, as shown in Fig. (5). The patsnap system clusters similar patents according to the similarity of the patent contents. The category of patents surrounding COVID-19 included therapeutic area, detecting method, detecting devices, and protecting tools/devices/masks. There were three areas in the therapeutic area according to the patent 3D landscape, comprising Traditional Chinese medicines, chemical drugs, and vaccines. As shown in Fig. (5). “Peak 1”, “peak 2” and “peak 3”, represented traditional Chinese medicines, chemical drugs, and vaccines in the 3D landscape, respectively. “Peak 4” “Peak 5” and “Peak 6”, represent detecting methods, detecting devices/body temperature/artificial intelligence smart detection, and protecting tools/devices/masks, as shown in Fig. (5), respectively.
The largest IPC classification is A61P31, which indicates that the therapeutic area plays a leading role in patent technologies surrounding COVID-19, as shown in Figs. (5-6). Applicants from China are major contributors in the therapeutic area (Fig. 6). The top 10 applicants from China contribute to the therapeutic area as well, as shown in Fig. (7). Applicants from India are almost not involved in the therapeutic area surrounding COVID-19 (Fig. 6). Applicants from India mainly contribute to body temperature detecting devices/tools surrounding COVID-19. Applicants from China also contributed to the detection method development surrounding COVID-19, as shown in peak 6 in 3D landscape.
2.3. Technology Evolution
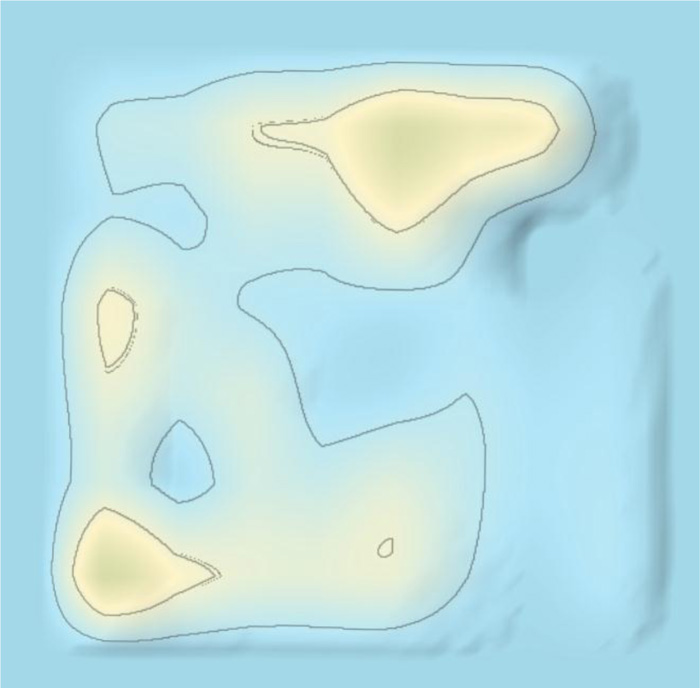
As shown in Fig. (5), the main therapeutic strategy was presented by red dots in the 3D landscape, which covered areas of traditional Chinese medicines, chemical drugs, and vaccines. As shown in Fig. (8), the evolution of 3D landscape showed that traditional Chinese medicines and chemical drugs were the fast developed at the early stage of the outbreak of COVID-19. The development of vaccines was much later than the development of traditional Chinese medicines and chemical drugs (Fig. 9). Vaccines-related technologies started to be clustered after Jun 2020 in Patent 3D landscapes. This indicated that it needed about half a year to develop vaccine-related technologies against COVID-19. However, it just needs about one week to make a Chinese medicine prescription against COVID-19 according to the patient’s clinic characteristics. This was the reason patents surrounding traditional Chinese medicines were rapidly appeared and clustered in the 3D landscapes.
In addition, the significant feature against COVID-19 in the 3D landscape appeared numerous traditional Chinese medicines. The number of patents surrounding traditional Chinese medicines is more than the number of patents surrounding chemical drugs (Fig. 5).
3. RESULTS AND DISCUSSION
Traditionally, Europe is the critical patent area in technology. However, we only found a few European patent applications surrounding COVID-19. Therefore, we did not include the analysis of European patents in this article. It may be due to some unpublished patent documents. Obviously, it is certain that China, India, and the US have the advantage in patents surrounding COVID-19.
Our study showed the patent landscape surrounding COVID-19 from six major countries for the first time, as shown in Fig. (5). The patent surrounding COVID-19 comprised therapeutic area, detecting method, detecting devices/body temperature/artificially intelligent detection, and protecting tools/devices/masks. Specifically, the therapeutic area comprised traditional Chinese medicines, chemical drugs, and vaccines. Our study showed the number of patents in the therapeutic area was about half in all patent applications, which indicated that therapeutic approaches, detecting and protecting approaches were the same in importance against COVID-19. This is a significant feature for COVID-19, compared to anti-cancer or anti-osteoporosis. Almost no patents were surrounded the protecting approaches in anti-cancer or anti-osteoporosis therapeutic strategies [15, 16]. As shown in (Fig. 5). “Peak 1”, “peak 2” and “peak 3” was exclusively focused on the therapeutic area in the 3D landscape. “Peak 4” “Peak 5” and “Peak 6”, concerned in detecting methods, detecting devices/body temperature, and protecting tools/devices.
It was hard to find common characteristics for chemical drugs or vaccines against COVID-19. However, it was easy to find common features for traditional Chinese medicines against COVID-19. In essence, the patents with traditional Chinese medication focused on three aspects. On one hand, the traditional Chinese medicine composition showed its effects by lung-ventilating, dampness-clearing, cooling blood to prevent deterioration for patients with COVID-19. On the other hand, the traditional Chinese medicine compositions showed their effects by clearing away heat, detoxifying, eliminating phlegm, relieving cough, and reducing sputum to prevent and treat COVID-19. Other traditional Chinese medicine compositions showed their effects against COVID-19 by strengthening body resistance and rescuing the lung functions.
Technology evolution showed that at the early stage of the pandemic of COVID-19, patent technologies surrounding COVID-19 were mainly traditional Chinese medicines, chemical drugs, and detecting methods, which were almost simultaneously appeared, as shown in Fig. (8). Later, vaccine-related technologies were started to be emerged and developed, as shown in Fig. (9). Thus, developing vaccine is more or less later than developing chemical drugs and traditional Chinese medicines in therapy against COVID-19.
A practical approach against coronavirus diseases is to develop vaccines. However, traditional medicines showed to be emerged in therapy against COVID-19. Also, It has been shown that traditional Chinese medicines are safe and effective in the treatment of COVID-19 [17-20]. Thus, traditional Chinese medicines could play an emergency role against the pandemic while a novel pandemic is emerging. Traditional Chinese medicines have thousands of years of history in the clinic, with prescription flexible in using compositions of traditional Chinese medicines, which are an essential foundation contributed to against COVID-19. Traditional Chinese medicines can play a unique role in a special period. Notably, at the early stage of the outbreak of COVID-19, there were no chemical drugs or vaccines available for the treatment of COVID-19. Traditional Chinese medicines definitely played a pioneering role in China in therapy against COVID-19. This may be the reason that the mortality rate of COVID-19 is very low in China.
The molecular mechanism of action of Traditional Chinese medicines against COVID-19 is not clear. However, the mechanism of action from some natural products from herbal medicine has been studied. Basically, those nature products act in the virus attachment approach and RNA replication process [21-29]. Uncovering the mechanism of action of natural products against coronavirus provides more scientific illustration and evidence for effectivity of Traditional Chinese medicines against COVID-19.
China National Medical Products Administration received 1012 domestic registration applications in chemical drugs, 7 domestic registration applications in traditional Chinese medicines, and 57 domestic registration applications in biological products in 2020 [30]. Over 90% of registration applications are from chemical drugs. Chemical drugs are the mainstream against diseases, e.g., cancer and osteoporosis [16]. However, so far for coronavirus, the effective approach against the virus is vaccines or traditional Chinese Medicines, but not chemical drugs. This indicates that chemical drugs are pretty rare to be successfully developing against coronavirus disease. Thus, a rapid therapeutic strategy against coronavirus disease is to develop vaccines. Another good therapeutic strategy against coronavirus disease is to develop traditional Chinese Medicines. Developing chemical drugs against coronavirus disease may be time-consuming, low effective, and not easy to be a successful approach.
So far, some COVID-19 vaccines have been developed by Pfizer, Sinovac, Sinopharm, and AstraZeneca. A recent study showed that COVID-19 vaccine efficacy was 72.8% -78.1%, compared with the alum-only group [31]. Currently, the vaccines provide the most effective approach against COVID-19. Developing vaccines is an effective strategy against COVID-19. The US FDA has approved the first COVID-19 vaccine in August 2021, known as the Comirnaty [32]. In December 2020, the US FDA issued an emergency use authorization (EUA) for Moderna COVID-19 Vaccine [33]. In February 2021, the US FDA issued an emergency use authorization for the third vaccine for Janssen COVID-19 Vaccine [34].
In the future, developing new vaccine technologies will be significant in therapeutic strategy against COVID-19 or other coronaviruses. Notably, such as developing mRNA vaccines, mRNA vaccines give that recipe to the immune system, instead of presenting the body with the deadly virus. The process is simpler and more robust, dose production is faster. As technology developing of mRNA vaccines, it will provide a quicker and more straightforward approach against COVID-19 or other coronaviruses. Emerging of new technology will provide a quicker approach for human beings against COVID-19 or other coronaviruses.
CONCLUSION
China is the most prolific country in patents surrounding COVID-19, with over 300 published patent documents. The primary therapeutic strategies against COVID-19 comprise traditional Chinese medicines, chemical drugs, and vaccines. A practical approach against COVID-19 is to develop vaccines. A significant difference in therapeutic strategies against COVID-19 is the contribution of traditional Chinese medicines. Traditional Chinese medicines can play an emergency or pioneer role in against novel diseases, particularly in unknown coronavirus, while an unknown disease has emerged. An effective and fast approach against COVID-19 is to use vaccines or traditional Chinese Medicines. The present study showed the development of therapeutic strategy surrounding COVID-19 based on patent insight for the first time, and provides new insight on therapeutic strategy against COVID-19.
CURRENT AND FUTURE DEVELOPMENTS
Traditional Chinese medicines or Natural compounds provide a promising frontline in the fighting against COVID-19. Traditional Chinese medicines may be a therapeutic strategy for treating patients with COVID-19. However, it still needs more studies for the mechanism of action of traditional Chinese medicines. It is a feasible approach to uncover the mechanism of action and targets the natural products isolated from traditional Chinese medicines. This treatment of traditional Chinese medicines has provided benefits, including lower side effects, achieving multiple therapeutic targets, and reducing the likelihood of resistance.
Natural products from traditional Chinese medicines may be a new trend in developing drugs against the COVID-19 in the future. Natural products from traditional Chinese medicines with low or no toxicity for humans promise therapeutic strategies against the COVID-19.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
