All published articles of this journal are available on ScienceDirect.
A Negative History of Epidemiologic and Demographic Factors Associated with High Numbers of Covid-19 Deaths
Abstract
Background:
Substantial differences between countries have been observed in terms of Covid-19 death tolls during the past two years. It is of interest to find out how the epidemiologic and/or demographic history of the population may have had a role in the high prevalence of the Covid-19 in some countries.
Objective:
This observational study aimed to investigate possible relations between Covid-19 death numbers in 39 countries and the pre-pandemic history of epidemiologic and demographic conditions.
Methods:
We sought the Covid-19 death toll in 39 countries in Europe, America, Africa, and Asia. Records (2019) of epidemiologic (Cancer, Alzheimer's disease) and demographic (natality, mortality, and fertility rates, percentage of people aged 65 and over) parameters, as well as data on alcohol intake per capita, were retrieved from official web pages. Data were analysed by simple linear or polynomial regression by means of Microsoft Excell software (2016).
Results:
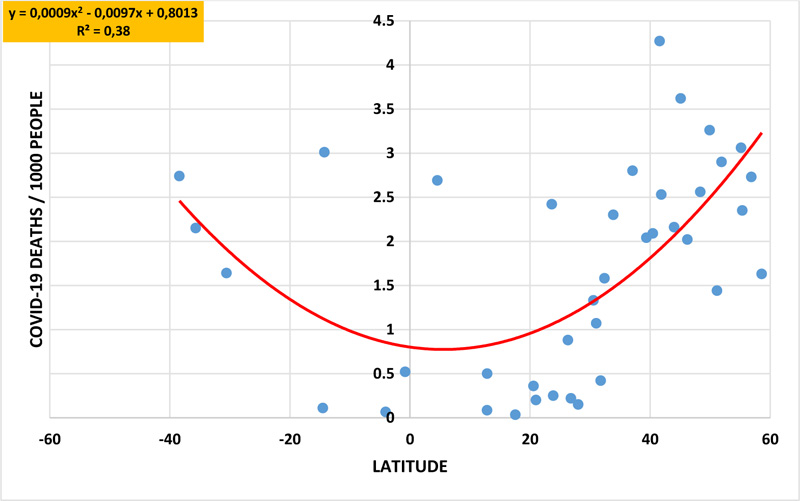
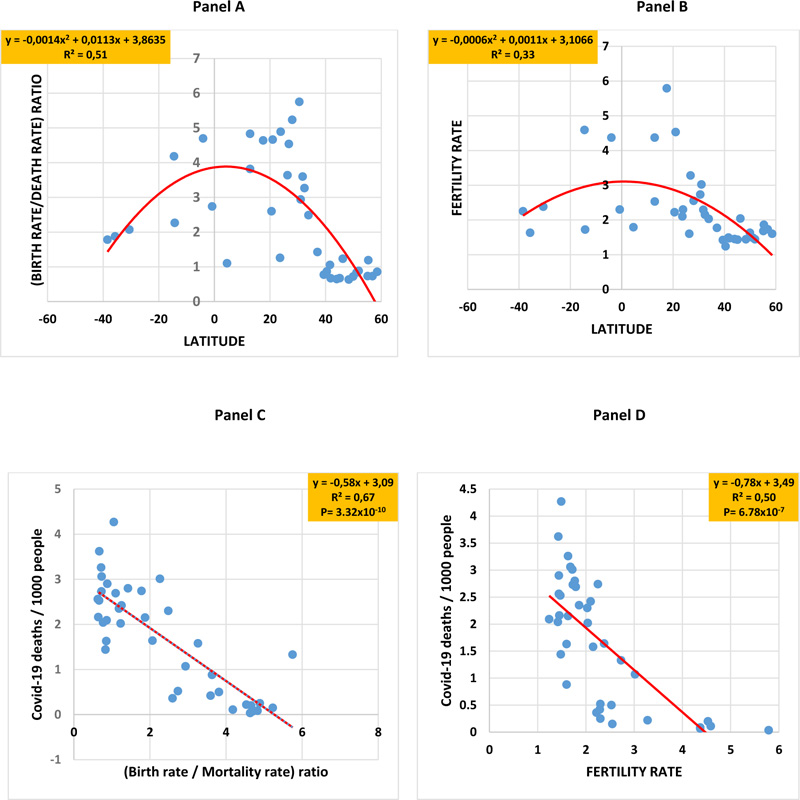
When Covid-19 death numbers were plotted against the geographic latitude of each country, an inverted bell-shaped curve was obtained for both the first and second years (coefficient of determination R2=0.38) of the pandemic. In a similar manner, inverted bell-shaped curves were obtained when latitudes were plotted against the scores of cancer plus Alzheimer's disease (R2 = 0,65,), the percentage of advanced age (R2 = 0,52,), and the alcohol intake level (R2 = 0,64,). Covid-19 death numbers were positively correlated to the scores of cancer plus Alzheimer's disease (R2= 0.41, P= 1.61x10-5), advanced age (R2= 0.38, P= 4.09x10-5), and alcohol intake (R2= 0.48, P= 1.55x10-6). Instead, bell-shaped curves were obtained when latitudes were plotted against the birth rate/mortality rate ratio (R2 = 0,51) and the fertility rate (R2 = 0,33). In addition, Covid-19 deaths were negatively correlated with the birth rate/mortality rate ratio (R2= 0.67) and fertility rate (R2= 0.50).
Conclusion:
The results show that the 39 countries in both hemispheres in this study have different patterns of epidemiologic and demographic factors, and that the negative history of epidemiologic and demographic factors of the northern hemisphere countries as well as their high alcohol intake were very correlated with their Covid-19 death tolls. Hence, also nutritional habits may have had a role in the general health status of people in regard to their immunity against the coronavirus.
1. INTRODUCTION
The Covid-19 pandemic was declared two years ago following high death tolls caused by SARS-CoV-2 infection around the world [1]. The virus and its target human cell receptor ACE-2 were identified [2, 3]. The understanding of the pathophysiology of Covid-19 [4-8] has allowed rational therapeutic management of the disease. For inflammatory conditions (cytokine storms), infections, and microcoagulations, patients were primarily treated with anti-inflammatory corticoids, antibiotics, and blood thinners [9, 10]. Vitamins (vitamin C, vitamin D), minerals (zinc and magnesium) and probiotics/prebiotics products were used as well to boost the immune system [11-13]. High scores of mortality were significantly associated with several risk factors, such as advanced age, overweight, cardiovascular and neurovascular diseases, chronic renal failure, diabetes, and cancer [14-16].
The large differences in Covid-19 mortality between countries led to questions regarding the possible causes. Africa and Asia have suffered less Covid-19 incidence and mortality [17]. Many researchers have hypothesized that Covid-19 prevalence is influenced by the immune status of an individual [18, 19]. On the other hand, alcohol consumption is known to be associated with several diseases, such as cancer [20-22]. Hence, nutritional habits may have key relevance in the course of a disease. For instance, a significant difference can be found in the nutritional habits between African countries and occidental countries, which certainly may affect the composition of intestinal microbiota, and consequently, part of the immune capacity of people. The purpose of this observational study was to examine Covid-19 deaths in 39 countries after two years of the pandemic to see if any specific epidemiologic or demographic factors could explain the differences in Covid-19 prevalence.
2. MATERIALS AND METHODS
We obtained Covid-19 death numbers from official web pages that follow Covid-19 mortality around the world [23, 24]. On the other hand, epidemiologic data [25], demographic data [26], and alcohol intake per capita [27] corresponding to 2019 were obtained from officially published documents. The data, correlation parameters, linear and polynomial regression, and figures were processed and analyzed by the Microsoft Office Excel software (2016).
The cancer score was estimated as the number of cancer cases reported in the table of ten most common causes of death in each country [25]. The Alzheimer's disease score was estimated as ‘11 minus the ranking order of Alzheimer's disease among the 10 causes of death in each country’ [25]. If Alzheimer's disease was not listed, the score was ‘0’. The advanced age score was estimated as the percentage of people aged 65 years and over. The alcohol intake score was expressed as liters/capita for people aged 15 years and over [27]. The data and records were carried out according to the STROBE guidelines [28].
3. RESULTS
We checked the geographic (latitude and longitude) coordinates of 39 countries, distributed as follows: America (the United States and Mexico), South America (Brazil, Colombia, Argentina, and Chile), Europe (France, Italy, Spain, Germany, Portugal, United Kingdom, Estonia, Lithuania, Croacia, Serbia, Poland, Romania, North Macedonia, Latvia, and Ukraine), and Africa (Morocco, Argelia, Mauritania, Tunisia, Egypt, Sudan, Congo, and Senegal). The population of the above countries is a sample of 3525 million people, which represents 44.43% of the total world population. 32 of the above countries are located in the northern hemisphere, where most (about 90%) of the world's population lives.
When the geographic latitude of each country was plotted against the Covid-19 death numbers, the polynomial regression analysis generated inverted bell-shaped curves for both the first and second year (R2= 0.38) of the pandemic (Fig. 1). Higher death rates were recorded at higher geographic latitudes in both hemispheres, like in European countries. Instead, lower death rates were recorded in countries located near the equator or those located at lower latitudes, like many African countries. The geographic longitude did not have any correlation with climate nor with other factors of the study.
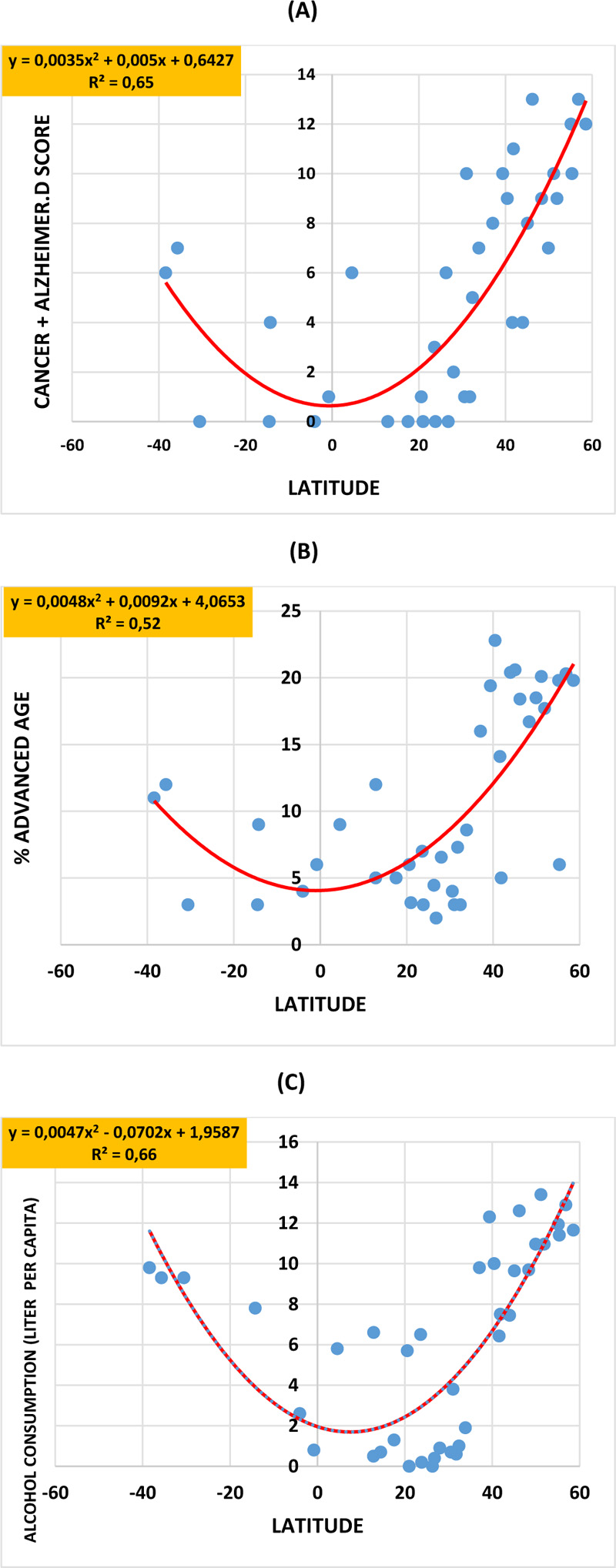

In a similar manner, inverted bell-shaped curves were obtained when the geographic latitudes were plotted respectively against the scores of cancer plus Alzheimer's disease (R2 = 0,65) (Fig. 2 panel A), the percentage of advanced age (R2 = 0,52) (Fig. 2 panel B) and the alcohol intake level (R2 = 0,64) (Fig. 2 panel C). Interestingly, Covid-19 death numbers significantly positively correlated to the scores of cancer plus Alzheimer’s disease (R2= 0.41, P= 0.61x10-5), advanced age (R2= 0.38, P= 4.09x10-5) and alcohol intake (R2= 0.48, P= 1.55x10-6) (Fig. 3, panels A-C), respectively. Instead, bell-shaped curves were obtained when the geographic latitudes were respectively plotted against the birth rate/mortality rate ratio (R2 = 0,51) (Fig. 4, panel A) and the fertility rate (R2 = 0,33) (Fig. 4, panel B), with the lower values being recorded in countries located at higher latitudes, like in European countries. Interestingly, Covid-19 deaths significantly and highly negatively correlated to the birth rate/mortality rate ratio (R2 = 0,67, P= 3.32x10-10) and the fertility rate (R2 = 0,50, P= 6.78x10-7) (Fig. 4, panels C, D).
In an attempt to model the relationship between the epidemiologic and demographic history of the population of the 39 countries included in the study, along with both their geographic latitude and their Covid-19 deaths, we aimed to develop a global parameter that takes into account several epidemiologic and demographic factors of the present study. The parameter is called the general immune capacity (GIC) score, which we have expressed as follows:
GIC = (birth rate/mortality rate) x fertility rate / (cancer sore + Alzheimer's disease score) + (% age 65 years and over) + (liters of alcohol intake per capita per year).
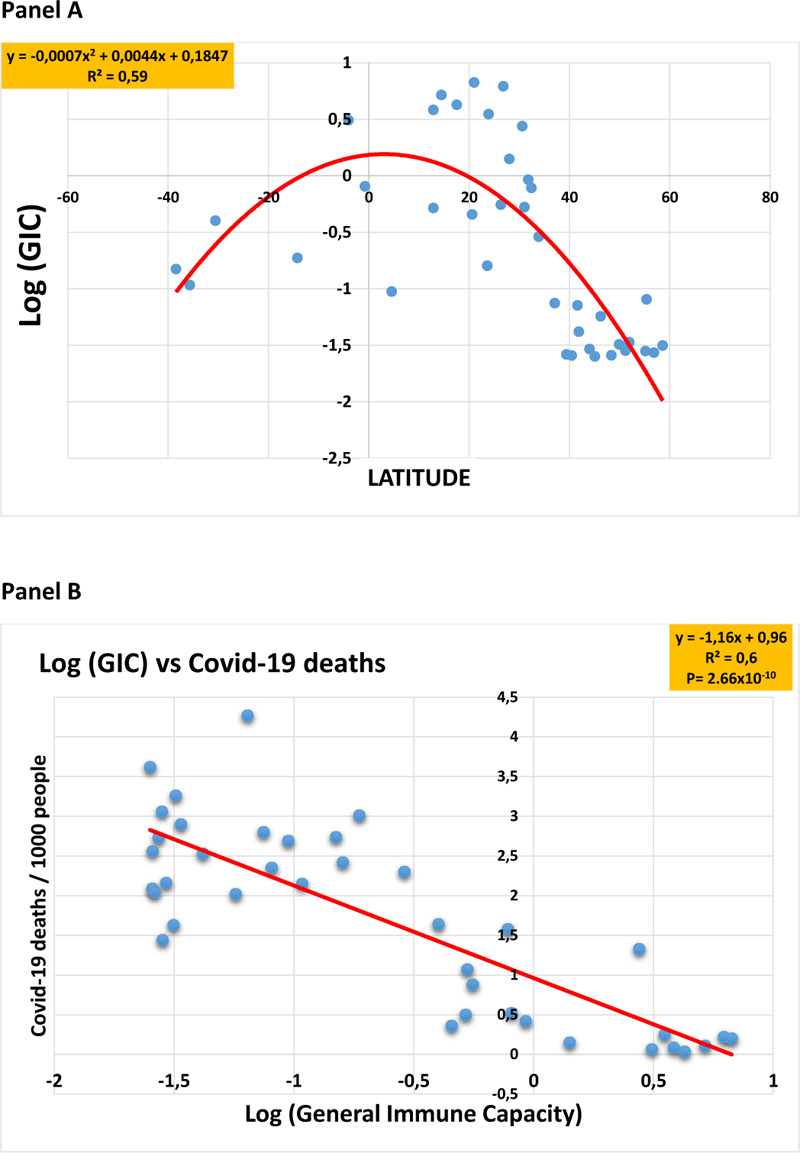
When the geographic latitudes were plotted against the GIC scores (expressed in logarithm units) of each country, the polynomial analysis generated a bell-shaped curve (R2 = 0.59) (Fig. 5, panel A), and the GIC values were found to highly correlate (R2= 0.67, P= 2.66x10-10) to the Covid-19 deaths (Fig. 5, panel B).
4. DISCUSSION
The low COVID-19 prevalence in northern and central Africa compared to Europe during the pandemic's first two years was of particular interest. Questions have been raised about the reasons behind such significant differences. In the present observational study, we have analysed epidemiologic and demographic history as well as records of alcohol intake in 39 representative countries from around the world. Indeed, the population of the countries in this study represented more than 44% of the total world’s population. In the present observational study, we have shown that some demographic factors vary from one country to another, and they are highly correlated with geographic latitude. Indeed, the birth rate/mortality rate ratio and the fertility rate are higher, for example, in African countries located around the equator or at low and median latitudes, such as Senegal, Mauritania, Morocco, and Egypt. Instead, countries located at higher latitudes, like those of Europe and America, have lower trends in birth rate/mortality rate ratio and fertility rate. On the other hand, the percentage of people aged 65 years and over is higher in countries of higher latitudes, mainly those of the northern hemisphere, in contrast to countries of lower latitudes, such as those located in Africa. Likewise, the above data point to the low prevalence of Covid-19 disease in countries that have a high percentage of young people, and most of them are located mainly at the lower and median geographic latitudes of both hemispheres. In contrast to northern hemisphere countries at higher latitudes, those at lower and median latitudes have lower scores for both cancer and Alzheimer's disease, and they also have low levels of alcohol consumption. The above results clearly indicate that the negative patterns of the epidemiologic and demographic history, as well as the consumption habits of excessive alcohol intake, all have a close positive correlation and relationship with the high death tolls in many countries, regardless of the vaccination share of the populations. In addition, our score of the general immune capacity (GIC), similarly to many demographic, epidemiologic, and alcohol intake factors, is very much correlated with both the geographic latitudes and the Covid-19 death patterns. The GIC score developed in this study could be a genuine human-related epidemiologic, demographic, and consumption habit factor for the estimation of the vulnerability of a given population against either Covid-19 or other non-communicable diseases. The immune capacity of an individual is related to nutritional habits. It is known that in Africa, many food recipes are based on natural plants and food products, such as fruits, seeds, dry fruits and tea [29-35], breastfeeding habits [36-39] and other healthy milk products [40-59], as well as many plant oils, like olive and argan oils, that have proven to be healthy against cardiovascular diseases and many chronic health conditions [60-74], and are consumed in traditional societies. The above food items are composed of hundreds of molecular ingredients that are key elements for healthy nutritional habits that contribute to the body’s anti-oxidant status and the general immune capacity. All of the above nutritional parameters are of high value for boosting the immune system, and might represent a relevant parameter against chronic and infectious diseases.
The strength of our study is the significant statistical result that exhibits a possible link between the burden of chronic diseases, such as cancer and Alzheimer's disease, the rate of the elderly and alcohol consumption in a country with the level of covid-19 deaths, as well as the inverse correlation between the birth rate in a country and the number of covid-19 deaths. These parameters have pointed to Western countries as a privileged target for the coronavirus. The weakness of our study is the lack of more precise data on the exact demographic and epidemiological characteristics of the people counted as having died from the coronavirus among the populations of the 39 countries analyzed in our study.
CONCLUSION
The data from the present study support the hypothesis that the negative epidemiologic and demographic history of countries of higher latitudes may play a role in the high COVID-19 death tolls in many European countries. In the above countries, heavy drinkers may also have had a bad outcome from coronavirus infection.
LIST OF ABBREVIATIONS
| GIC | = General Immune Capacity |
| AD | = Alzheimer's Disease |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
STROBE guideline has been followed.
AVAILABILITY OF DATA AND MATERIALS
Covid-19 death tolls during the past two years were investigated in 39 countries in regard to epidemiologic and demographic factors.
The data will be avalable at, https://dataverse.harvard.edu/ dataset.xhtml?persistentId=doi:10.7910/DVN/XWOREU
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


