All published articles of this journal are available on ScienceDirect.
The Frequency and Severity of Post-vaccination Reactions after Vaccination against COVID-19 in Sudanese Health Workers in Khartoum Governmental Hospitals, 2021
Abstract
Background
COVID-19 has been ascribed to the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the disease has a wide spectrum of clinical manifestations varying from asymptomatic, minor flu-like symptoms to acute respiratory distress syndrome (ARDS), pneumonia and death. Vaccinations against COVID-19 are counted to be of great significance to prevent and control COVID-19. This study aims to verify the actual frequency of vaccine reactions after vaccination against COVID-19 and their severity.
Materials and Methods
A descriptive cross-sectional study was conducted from July to September 2021 in Three of Khartoum’s government hospitals. The study population included Sudanese health workers who received two doses of COVID-19 vaccinations. Data was entered and analyzed using SPSS version 24.
Results
This study covered 200 participants, who received two doses of the COVID-19 vaccine, of them, 129 (64.5%) were female, with a mean age of 33.13 ± 5.860 (mean ± SD) years. The male mean age was 35.59 ± 7.996 years. Pain is the most common local reaction that occurs in participants with 73.0%. Unusual fatigue, fever, and headache showed the most systemic post-vaccination reactions that occurred among participants, with percentages of 56.5%, 43.0%, and 34.5%, respectively. There is an insignificant association between having a previous COVID-19 infection and developing local and systemic post-vaccination reactions. Also, comorbidities appeared to have an insignificant association with developing local or systemic post-vaccination reactions. Systemic post-vaccination reactions showed a significant association with participants need for post-vaccination medical care.
Conclusions
The majority of Sudanese health workers received AstraZeneca Company COVID-19 and showed local and systemic post-vaccination reactions that did not need medical care in most of them. Results provide assurance about the high level of safety of COVID-19 vaccines.
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic arose in December 2019 and caused the coronavirus disease 2019 (COVID-19). As yet, COVID-19 has infected over one million people worldwide, and deaths have exceeded two million [1]. COVID-19 has led to a wide range of clinical manifestations, from no symptoms at all and minor flu-like symptoms to severe conditions like acute respiratory distress syndrome (ARDS), pneumonia, and death. Controlling the COVID-19 pandemic is anticipated through measures such as hand washing, hand sanitizing, avoiding touching face with unwashed hands, respiratory hygiene, social distancing, mask wearing, testing and contact tracing, travel restriction, new antiviral drugs, effective vaccine and public education. While it’s feasible to achieve herd immunity by gaining natural immunity through infections, the resulting death toll and its consequences would be devastating [2].
Immunization is one of the most effective and economical health strategies for preventing infectious diseases. Consequently, COVID-19 vaccines are consi- dered crucial for preventing and controlling the spread of the virus [3]. To date, developing a new vaccine has been a lengthy process, usually taking between 10 and 15 years. The Mumps vaccine is considered the fastest vaccine that has been developed and approved for use, as it took approximately 5 years [4]. Therefore, developing a COVID-19 vaccine within 12–24 months is clearly a significant challenge. The UK's Medicines and Healthcare products Regulatory Agency has granted emergency use authorization to three COVID-19 vaccines: the Pfizer-BioNTech mRNA vaccine (BNT162b2), the Oxford-AstraZeneca adenovirus-vectored vaccine (ChAdOx1 nCoV-19), and the Moderna mRNA vaccine (mRNA-1273) [5]. However, there are also worries about potential vaccine reactions. The rate of adverse events caused by vaccines is generally low, varying from 4.8 to 83.0 per 100,000 doses for commonly administered vaccines. The exact number of true allergic reactions to the usual vaccines is not precisely known; but is estimated to range from one per 500,000 to one per 1 million doses for most vaccines. All three types of COVID-19 vaccines can induce a wide range of vaccine-related adverse reactions. The most frequent side typically arises from generalized inflammation triggered by the vaccine and is generally localized to the injection site [6].
2. METHODS
This is a descriptive cross-sectional study conducted from July to September 2021. The study was conducted in Khartoum state at three different Khartoum governmental hospitals. The samples were collected from 200 Sudanese health workers (134 from two hospitals and 66 samples from the third hospital) including medical doctor, nurses, pharmacist, laboratory scientists and nutritionists whether they had patient contact who received 2 doses of COVID-19 vaccinations. ; The selection of 200 HCW was based on a power analysis designed to achieve 80% power to detect significant post-vaccination reactions with an alpha level of 0.05. This sample size ensures representativeness across participating hospitals and reflects the demographic diversity of healthcare workers in Khartoum. [7]
Those who received one dose of vaccination were excluded.
Data was collected using a structured questionnaire. The questionnaire was filled out by the participant to obtain basic information in addition to social character- istics regarding COVID-19, such as being test-confirmed and unconfirmed with COVID-19; and post-vaccination reactions to vaccination symptoms.
Data was analyzed by computer using the statistical package for social science (SPSS) software version 24, as appropriate. P<0.05 is considered statistically significant (confidence interval: CI 95%). Descriptive statistics (mean, standard deviation, and range) were used for the present data. Correlations between the variables were determined using the Chi-Square test. The results were expressed in (Tables 1-11 and Figs. 1 and 2).
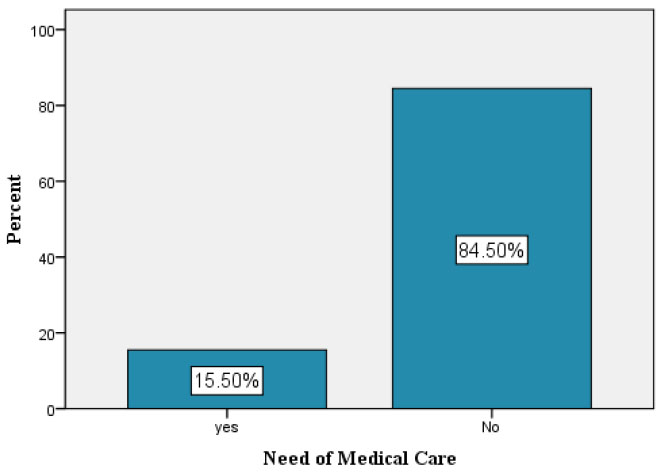
Percentages of participants needed for medical care.
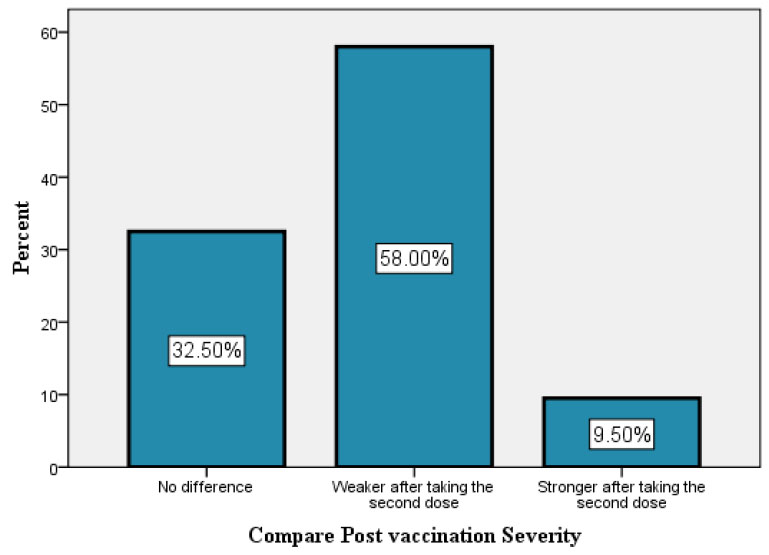
Percentages of different post vaccination complication severity.
3. RESULTS
This study covered 200 participants who received two doses of COVID-19 vaccine. Of them, 129 (64.5%) were female, with a mean age of 33.13 ± 5.860 (mean ± SD) years. The male mean age was 35.59 ± 7.996 years.
Only 18.5% of participants had a history of previous COVID-19 infection, and 81.5% of them tested because of developing symptoms.
The majority of participants, 81%, received the AstraZeneca Company COVID-19 vaccine, while only 17.5% received the Pfizer company vaccine.
Pain is the most common local reaction that occurs in participants with 73.0%.Tenderness and warmth were the second most common local reactions, with 44.0% and 30.0%, respectively. 16% showed no local reactions.
Unusual fatigue, fever, and headache showed the most systemic post-vaccination reactions that occurred among participants, with percentage 56.5%, 43.0%, and 34.5%, respectively. 29.5% expressed no systemic reaction.
A Chi-Square test was conducted as displayed in Table 1 and showed a statistically insignificant association between having a previous COVID-19 infection and developing post-vaccination reactions (P = 0.379).
Being affected by comorbidity appeared to have insignificant association with developing local or systemic post-vaccination reactions (P = 0.813, .0.158) respectively, as detailed in Tables 2 and 3.
Moreover, age groups have an insignificant association with developing local or systemic post-vaccination reactions (P = 0.110, 0.672) respectively, as revealed in Tables 4 and 5.
There is significant association between gender and developing of post-vaccination reactions (P = 0.002), as detailed in Table 6, Research indicate that females often exhibit that stronger immune response due to hormonal influences, which my lead to a higher prevalence of combined local and systemic reactions post vaccination. [8].
| - | Post Vaccination Reaction | Total | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| Local Post Vaccination Reaction | Systemic Post Vaccination Reaction | Both (local and systemic) | None (no local neither systemic) | |||||
| Get Covid | Yes | Count | 11 | 2 | 21 | 3 | 37 | 0.379 |
| % of total | 5.5% | 1.0% | 10.5% | 1.5% | 18.5% | |||
| No | Count | 28 | 10 | 107 | 18 | 163 | ||
| % of total | 14.0% | 5.0% | 53.5% | 9.0% | 81.5% | |||
| Total | Count | 39 | 12 | 128 | 21 | 200 | ||
| % of total | 19.5% | 6.0% | 64.0% | 10.5% | 100.0% | |||
Table 2.
| - | - | Having Comorbidity | P-value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Having local reactions | yes | Count | 40 | 128 | |
| % within having local reactions | 23.8% | 76.2% | 0.813 | ||
| % within having comorbidity | 85.1% | 83.7% | |||
| % of total | 20.0% | 64.0% | |||
| No | Count | 7 | 25 | ||
| % within having local reactions | 21.9% | 78.1% | |||
| % within having comorbidity | 14.9% | 16.3% | |||
| % of total | 3.5% | 12.5% | |||
| - | - | - | Having Comorbidity | P-value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Having systemic reaction | yes | Count | 37 | 104 | 0.158 |
| % within having systemic reaction | 26.2% | 73.8% | |||
| % within having comorbidity | 78.7% | 68.0% | |||
| % of total | 18.5% | 52.0% | |||
| No | Count | 10 | 49 | ||
| % within having systemic reaction | 16.9% | 83.1% | |||
| % within having comorbidity | 21.3% | 32.0% | |||
| % of total | 5.0% | 24.5% | |||
| - | - | - | Age Group | P-value | ||
|---|---|---|---|---|---|---|
| 20 to 30 | 31 to 40 | More than 40 | ||||
| Having local reactions | yes | Count | 55 | 88 | 25 | 0.110 |
| % within having local reactions | 32.7% | 52.4% | 14.9% | |||
| % within age group | 78.6% | 84.6% | 96.2% | |||
| % of total | 27.5% | 44.0% | 12.5% | |||
| No | Count | 15 | 16 | 1 | ||
| % within having local reactions | 46.9% | 50.0% | 3.1% | |||
| % within age group | 21.4% | 15.4% | 3.8% | |||
| % of total | 7.5% | 8.0% | .5% | |||
| - | - | Age Group | P-value | |||
|---|---|---|---|---|---|---|
| 20 to 30 | 31 to 40 | More than 40 | ||||
| Having systemic reaction | yes | Count | 50 | 71 | 20 | 0.672 |
| % within having systemic reaction | 35.5% | 50.4% | 14.2% | |||
| % within age group | 71.4% | 68.3% | 76.9% | |||
| % of total | 25.0% | 35.5% | 10.0% | |||
| No | Count | 20 | 33 | 6 | ||
| % within having systemic reaction | 33.9% | 55.9% | 10.2% | |||
| % within age group | 28.6% | 31.7% | 23.1% | |||
| % of total | 10.0% | 16.5% | 3.0% | |||
| - | - | - | Post Vaccination Reaction | Total | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Local Post Vaccination Reaction | Systemic Post Vaccination Reaction | Both (local and systemic) | None (no local neither systamic) | |||||
| Gender | Male | Count | 21 | 6 | 33 | 11 | 71 | 0.002 |
| % of total | 10.5% | 3.0% | 16.5% | 5.5% | 35.5% | |||
| Female | Count | 18 | 6 | 95 | 10 | 129 | ||
| % of total | 9.0% | 3.0% | 47.5% | 5.0% | 64.5% | |||
| Total | Count | 39 | 12 | 128 | 21 | 200 | ||
| % of total | 19.5% | 6.0% | 64.0% | 10.5% | 100.0% | |||
| - | - | - | Need of Medical Care | P-value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Having local reactions | yes | Count | 29 | 139 | 0.115 |
| % within having local reactions | 17.3% | 82.7% | |||
| % within need of medical care | 93.5% | 82.2% | |||
| % of total | 14.5% | 69.5% | |||
| No | Count | 2 | 30 | ||
| % within having local reactions | 6.2% | 93.8% | |||
| % within need of medical care | 6.5% | 17.8% | |||
| % of total | 1.0% | 15.0% | |||
| - | - | - | Need of Medical Care | P-value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Having systemic reaction | yes | Count | 29 | 112 | 0.002* |
| % within having systemic reaction | 20.6% | 79.4% | |||
| % within need of medical care | 93.5% | 66.3% | |||
| % of total | 14.5% | 56.0% | |||
| No | Count | 2 | 57 | ||
| % within having systemic reaction | 3.4% | 96.6% | |||
| % within need of medical care | 6.5% | 33.7% | |||
| % of total | 1.0% | 28.5% | |||
The majority of participants (84.5%) did not need any kind of medical care after receiving vaccination, while only 15.5% needed medical care (like over the counter medication e.g. Ibuprofen or acetaminofen, epinephrine auto injector, antihistamines, corticosteroids, Oxygen, perform diagnostic tests, cardiac evaluation and intervention).
There is no significant association between having local post vaccination reactions and the need for medical care (P = 0.115), as shown in Table 7. Systemic post-vaccination reactions showed a statistically significant association with participant needs for post-vaccination medical care (P = 0.002), as revealed in Table 8.
Developing local post-vaccination reactions is statistically significantly associated with the type of vaccine company received (P = 0.013), as shown in Table 9. No significant association was found between the type of vaccine company received and developing systemic post-vaccination reactions (P = 0.271), as shown in Table 10.
Post-vaccination complication severity experienced by participants: 32.5% experience no difference between two doses, 58% reveal weaker symptoms after the second dose, and the remaining 9.5% experience stronger symptoms after the second dose. Table 11 displayed a statistically significant association between vaccine company and post-vaccination complications severity (P = 0.006).
| - | - | Vaccine Company | P-value | |||
|---|---|---|---|---|---|---|
| Pfizer | AstraZeneca | Other | ||||
| Having local reactions | Yes | Count | 33 | 134 | 1 | 0.013* |
| % within having local reactions | 19.6% | 79.8% | .6% | |||
| % within vaccine company | 94.3% | 82.7% | 33.3% | |||
| % of total | 16.5% | 67.0% | .5% | |||
| No | Count | 2 | 28 | 2 | ||
| % within having local reactions | 6.2% | 87.5% | 6.2% | |||
| % within vaccine company | 5.7% | 17.3% | 66.7% | |||
| % of total | 1.0% | 14.0% | 1.0% | |||
| - | - | Vaccine Company | P-value | |||
|---|---|---|---|---|---|---|
| Pfizer | AstraZeneca | Other | ||||
| Having systemic reaction | yes | Count | 23 | 117 | 1 | 0.271 |
| % within having systemic reaction | 16.3% | 83.0% | .7% | |||
| % within vaccine Company | 65.7% | 72.2% | 33.3% | |||
| % of Total | 11.5% | 58.5% | 0.5% | |||
| No | Count | 12 | 45 | 2 | ||
| % within having systemic reaction | 20.3% | 76.3% | 3.4% | |||
| % within vaccine company | 34.3% | 27.8% | 66.7% | |||
| % of total | 6.0% | 22.5% | 1.0% | |||
| - | - | Compare Post Vaccination Severity | P-value | |||
|---|---|---|---|---|---|---|
| No Difference | Weaker after taking the Second Dose | Stronger after taking the Second Dose | ||||
| Vaccine Company | Pfizer | Count | 10 | 16 | 9 | 0.006* |
| % within vaccine company | 28.6% | 45.7% | 25.7% | |||
| % within compare post vaccination severity | 15.4% | 13.8% | 47.4% | |||
| % of total | 5.0% | 8.0% | 4.5% | |||
| AstraZeneca | Count | 53 | 99 | 10 | ||
| % within vaccine company | 32.7% | 61.1% | 6.2% | |||
| % within compare post vaccination severity | 81.5% | 85.3% | 52.6% | |||
| % of total | 26.5% | 49.5% | 5.0% | |||
| Other | Count | 2 | 1 | 0 | ||
| % within vaccine company | 66.7% | 33.3% | 0.0% | |||
| % within compare post vaccination severity | 3.1% | 0.9% | 0.0% | |||
| % of total | 1.0% | 0.5% | 0.0% | |||
4. DISCUSSION
This research examined the frequency and severity of post-vaccination reactions after vaccination against COVID-19 comprehensively. We try to find the actual consequences of receiving COVID-19 vaccines to provide reassurance and helpful information regarding what health care providers and vaccine recipients might expect after vaccination.
In the present study, the occurrence of post-vaccination reactions was assessed in 200 participants. 129 (64.5%) were female, with a mean age of 33.13 ± 5.860 (mean ± SD) years, and 71 (35.5%) were male, with a mean age of 35.59 ± 7.996 years, with an age range of (22-65) years. This is in consensus with the Arroliga et al. study that assessed allergic reactions and adverse events associated with the administration of mRNA-based vaccines in the same age range and female predominance [9].
The study finds that pain is the most common local reaction that occurs in participants (73.0%).Tenderness and warmth were the second most common local reactions, and 16% showed no local reactions. This result is in agreement with the findings of studies that illustrated that tenderness and local pain around the injection site were the most frequently reported local effects [5, 10, 11]. Additionally, our study showed that unusual fatigue, fever, and headache showed the most systemic post-vaccination reactions that occurred among participants. These results were also supported by studies that illustrated the most common systemic side effect was fatigue (62.2%), followed by headache (45.6%) and muscle pain (37.1%) [5, 10, 11].
The result presented in this research elaborates that age groups have an insignificant association with developing local or systemic post-vaccination reactions. This partially goes with the study that found the 20-60 years-old group experienced all the adverse events post-vaccination, whereas participants >60 years of age did not experience a few adverse events [12].
This study demonstrated that 18.5% of participants had a history of previous COVID-19 infection; they developed local and systemic post-vaccination reactions with no significant differences from those who did not have a previous COVID-19 infection. The results contradict the claims of the Pfizer vaccine recipients study in that the local reactions were similar, but they observed more frequent systemic side effects with a higher severity grade in patients with a previous COVID-19 infection [13].Also, the Andrea Ossato et al. study reveals that the number of participants who had previously been infected with COVID-19 experienced some side effects after receiving the first dose of the vaccine significantly higher compared with participants who had not previously been infected. On the other hand, the number of participants who experienced some side effects after the second dose and had previously been infected with COVID-19 was significantly lower compared with participants who had not previously been infected [11]. Therefore, these results should take into account the type of vaccine, as the majority of our study participants received the AstraZeneca vaccine.
Furthermore, the results revealed that post-vaccination reactions severity diversified between no differences between two doses (32.5%) and revealed weaker symptoms after the second dose (58%), while the remaining 9.5% experienced stronger symptoms after the second dose. This is in contradiction to the Abanoub Riad et al. data, which displayed that the prevalence of local and systemic side effects was higher among the participants who received two doses compared to the participants with one dose of the Pfizer vaccine [10].This opposition could be explained by the statistically significant association between vaccine company and post-vaccination complications severity in our results, as the majority received the AstraZeneca vaccine.
Furthermore, the result revealed that being affected by comorbidity appeared to have an insignificant association with developing local or systemic post vaccination reactions (P = 0.813, 0.158) respectively. In contradiction, an Iraqi study revealed that those with comorbid diseases such as hypertension, diabetes, asthma, arthritis, etc. and AstraZeneca vaccine receivers were statistically significant risk factors for having adverse reactions post-vaccination [14].
Only systemic post-vaccination reactions showed a statistically significant association with participant needs for post-vaccination medical care. This result was replicated in a similar study done in Nepali health workers and showed paracetamol seems to be required with the Oxford vaccine compared to Pfizer or Moderna vaccines to resolve the individuals' common symptoms [15].
The generalizability of the results is limited by the small sample size of the study population, so there should be more studies on a large population receiving COVID-19 vaccines. In addition, future studies should take into account the number of subjects with a history of previous SARS-CoV-2 infection and also continue to monitor the COVID-19 vaccine over a long period of time to detect novel and serious side effects.
CONCLUSION
The majority of Sudanese health worker participants received the AstraZeneca company COVID-19 vaccine. Pain is the most common local reaction, while unusual fatigue, fever, and headache showed the most systemic post-vaccination reactions, with an insignificant association between having previous COVID-19 infections and developing local or systemic post-vaccination reactions. Also, comorbidity appeared to have an insignificant association with developing local or systemic post-vaccination reactions.
The majority of participants did not need any kind of medical care after receiving vaccinations. Systemic post-vaccination reactions showed a significant association with participant needs for post-vaccination medical care. Post-vaccination complication severity experienced by participants: 32.5% experience no difference between two doses, and 58% reveal weaker symptoms after the second dose.
AUTHOR'S CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ARDS | = Acute respiratory distress syndrome |
| mRNA | = Messenger RNA |
| SARS-CoV-2 | = Severe acute respiratory syndrome coronavirus |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical research committee of the Sudan Medical Specialization Board and the ethics review committee of Internal Medicine Council were approved the study.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committee and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from each participant prior to enrollment in the study.
