All published articles of this journal are available on ScienceDirect.
Case Reports: Safety, Tolerability, and Efficacy of 5-Aminolevulinic Acid Phosphate, an Inducer of Heme Oxygenase 1, in Combination with Sodium Ferrous Citrate for the Treatment of COVID-19 Patients
Abstract
Background:
The COVID-19 pandemic is the greatest life-threatening disaster currently facing the worldwide population. COVID-19 patients with concomitant diseases such as chronic obstructive pulmonary disease and cardiovascular problems quickly develop severe pneumonia with low arterial oxygen saturation and multiorgan failure, resulting in sudden death. These symptoms are caused by deadly inflammation that occurs in various organs.
Objective:
Various types of inflammation caused by RNA virus infection have been known to be manageable by the induction of heme oxygenase-1 (HO-1) in local tissues. HO-1 is also known to be a key enzyme for the suppression of RNA viral replication. Therefore, in addition to standard medical care for pneumonic viral infection, we have attempted to treat COVID-19 patients with a highly effective HO-1 inducer, 5-aminolevulinic acid phosphate, in combination with ferrous sodium citrate (5-ALA with SFC).
Methods:
5-ALA with SFC is a supplement formulation registered in Japan as food with functional claims. Six patients with typical symptoms of COVID-19 and some suspected COPD associated with heavy smoking were given oral administration of multiple doses of 5-ALA with SFC at the Maximum Tolerated Dose (MTD) for 3 to 7 days, followed by treatment with a lower amount of 5-ALA with SFC for 2 to 3 weeks.
Results and Conclusion:
Each patient's recovery time was considerably shorter than reported for patients who received only standard care for SARS-CoV-2 infection. The results confirm the safety, tolerability, and efficacy of 5-ALA with SFC as a therapeutic supplement for patients with acute-phase COVID-19.
1. INTRODUCTION
COVID-19, which is caused by SARS-CoV-2 viral infection, originated in Wuhan, China, in December 2019 and has since spread all over the world. Currently, as of March 16, 2021, more than 120 million people have been infected and more than 2.66 million people have died from COVID-19. Most of the casualties of COVID-19 pandemic were associated with severe pneumonia and multiorgan failure. Although over 4,000 clinical trials have been conducted for the treatment of COVID-19 patients according to the NIH registry (https://clinicaltrials.gov/ct2/results?cond=COVID-19), only a few drugs have been approved for COVID-19 therapy in the emergent pandemic situation. Although the vaccinations for COVID-19 have been quickly developed worldwide (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines), the pandemic seems to be not controlled in most of the countries due to the frequent mutations in SARS-CoV-2 genome RNA.
Typical symptoms of COVID-19 include fever, headache, sore throat, dry cough, fatigue, muscle and joint pain, malaise, runny nose, diarrhea, dyspnea, tachypnea, and low arterial oxygen saturation by pulse oximetry (SpO2). In addition, COVID-19 patients developed severe acute pneumonia, blood coagulation, and multiorgan failure, resulting in poor respiration and blood circulation [1, 2]. Some of the patients with concomitant disease conditions such as Chronic Obstructive Pulmonary Disease (COPD), diabetes, hypertension, and cardiovascular problems, quickly develop severe pneumonia, disseminated intravascular coagulation and multiorgan failure, resulting in sudden death [3]. Cardiovascular complications are rapidly emerging as a key threat in COVID-19 in addition to respiratory disease and endotheliitis, which appear to comprise the typical pathology of COVID-19 [4]. These disease conditions are mainly caused by severe local tissue inflammation following a cytokine storm induced by SARS-CoV-2 infection in bronchial epithelial cells and vascular endothelial cells in various organs, where angiotensin-converting enzyme-2 (ACE2), a specific receptor for the SARS-CoV-2 spike glycoprotein, is highly expressed and mediates SARS-CoV-2 infection in those cells [5, 6].
Replications of various pathogenic RNA and DNA viruses, including human immune deficiency virus, Zika virus, Ebola virus, Dengue virus, Herpes simplex virus, and hepatitis B virus, have been shown to be inhibited by heme oxygenase-1 (HO-1) - dependent molecular mechanisms [7-12]. HO-1 is also known to be a key enzyme of anti-inflammatory actions in tissues under various stress conditions [13, 14].
5-aminolevulinic acid (5-ALA) is a sole precursor of heme biosynthesis and produced in mitochondria of both animal and plant cells. The exogenous administration of 5-ALA enhances heme biosynthesis resulting in HO-1 induction by produced free heme molecules. 5-ALA in combination with sodium ferrous citrate (5-ALA with SFC) is known to be a potent inducer of HO-1, whereas 5-ALA alone does not efficiently induce HO-1 [15, 16]. Thus, we have been treating COVID-19 patients with a nutritional supplement of 5-ALA with SFC, which has been approved and registered as a food with functional claims, such as enhancement of metabolism and reduction of blood sugar level [17-21] and improvement of sleep quality [22] in Japan. Furthermore, the efficacy of 5-ALA with SFC has also been shown in terms of its ability to increase respiratory efficiency, resulting in the reduction of oxygen consumption and carbon dioxide production [23]. This may help COVID-19 patients who are experiencing respiration difficulties.
SARS-CoV-2 uses the cell surface serine protease, transmembrane protease serine 2 (TMPRSS2) for cellular infection mediated by the interaction between viral spike glycoprotein and ACE2 expressed on the host cell surface [24]. A couple of serine protease inhibitors have been identified as potential therapeutics for COVID-19 [24, 25]. Because those serine protease inhibitors, such as camostat mesilate and nafamostat mesilate, are approved medications for pancreatitis treatment in Japan, one of these serine protease inhibitors was also employed in our clinical investigation. In this case report, we demonstrate the prevention of disease progression and the significant improvement in the recovery process of COVID-19 patients using an oral administration of 5-ALA with SFC and the complete recovery of all the patients treated with 5-ALA and SFC.
2. MATERIALS AND METHODS
2.1. Inclusion Criteria and Informed Consent
Six patients who visited Hanzomon Clinic for diagnosis and satisfied with the following 3 criteria were included in the present study:
1) COVID-19 patient with pneumonia determined by CT scan, and 2) COVID-19 patient with clinical symptoms & signs, elevated CRP and decreased SpO2, 3) COVID-19 patient who was diagnosed as mild or severe.
In this study severe patients were defined as those who complained of dyspnea, or those who required O2 inhalation irrespective of assist ventilation. COVID-19 patients free from dyspnea were defined as mild. Critical cases indicate the patients in whom sufficient O2 saturation could not be obtained despite O2 administration provided even under assist ventilation. None of the critical cases were enrolled.
Administration of 5-ALA with SFC, favipiravir, chloroquine, ciclesonide, and camostat described in this paper was approved by the ethical committee of Hanzomon Gastrointestinal Clinic, and the informed consent was obtained from the patients or his proxy (Case 1). This research was conducted in accordance with the Helsinki Declaration. CARE guidelines were also followed for this study.
2.2. 5-Aminolevulinic Acid Phosphate (5-ALA)
5-ALA and SFC were enclosed in a capsule and administered orally to all patients, except for Case 1, for whom the contents of the capsule were dissolved in water and administered via a nasogastric tube. One capsule contains 25 mg 5-ALA and 28.7 mg SFC. These capsules are available as ALAplus Tou-Down Rich from SBI ALApromo, Tokyo, Japan. When the dose of 5-ALA was altered, the dose of SFC was also changed accordingly in the same proportion. The dose of 5-ALA was determined depending on the clinical severity. When the patient was recognized as severe, the initial dose was set to 1,500 mg/day (500 mg t.i.d., approximately 25 mg/kg of body weight). In mildly affected patients, the initial dose was set to 750 mg/day (approximately 12.5 mg/kg of body weight). Thereafter, the dose was altered according to the clinical symptoms and data.
2.3. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Patient’s nasal cavity and pharynx samples were obtained with cotton swabs and tested for SARS-CoV-2 using a RT-PCR test according to the national standard. Testing was conducted by local public health laboratories in Tokyo area using the procedure described by National Institute of Infectious Diseases, Japan [26].
2.4. Antibody Detection
To detect antibodies (IgM and IgG) against SARS-CoV-2, we used a rapid immunochromatographic test manufactured by Kurabo Industries Ltd. (Osaka, Japan). The sensitivity of IgM test was 82.58% and the specificity was 100%. The sensitivity of the IgG test was 76.38% and the specificity was 100%.
3. CASE PRESENTATION
3.1. Case 1
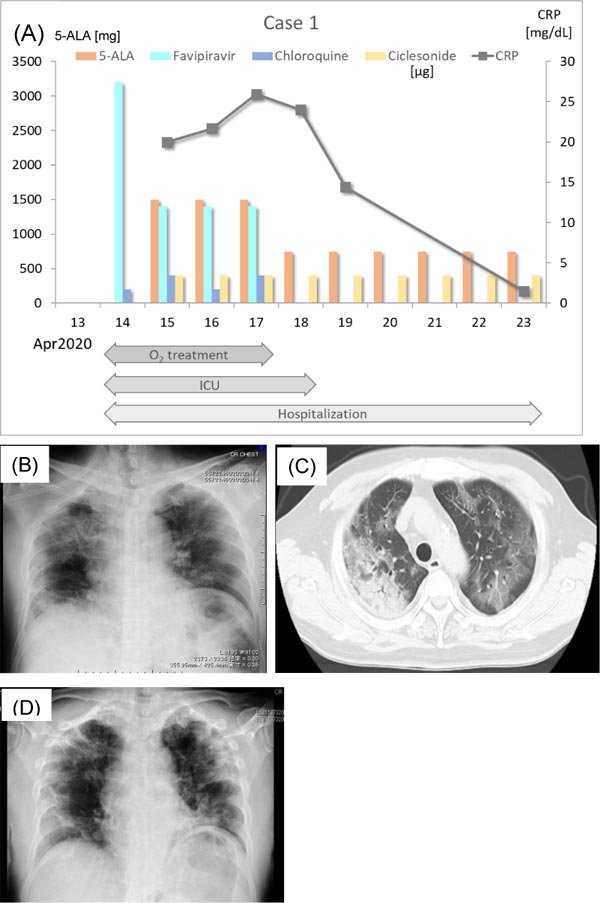
A 62-year-old man began to feel fatigued on April 3, 2020. He reported that before his symptoms developed, one of his office coworkers had received a positive RT-PCR result for SARS-CoV-2. The patient had a history of type 2 diabetes mellitus (HbA1c = 6.9%) and was being treated with oral hypoglycemic agents. On the day after fatigue symptoms started, he had a fever of 38.7 °C. He called the physician in charge, who prescribed an antipyretic (acetaminophen, 500 mg as needed). His fever temporarily decreased. However, fever (from 37.5 to 39°C) returned. His fever persisted for 8 days and was accompanied by cough, appetite loss, and dyspnea. He checked in to the Japanese Red Cross Musashino Hospital by ambulance on April 14, 2020 (Fig. 1A). On admission, he was transferred to the Intensive Care Unit (ICU) because of severe hypoxia and dyspnea on physical examination. Hypoxia resolved after oxygen inhalation, and his SpO2 was 95% with administration of 10 L/min nasal oxygen. Chest radiography and chest computed tomography (CT) scan revealed pneumonia in the right upper lobe and infiltrative shadow in the left hilum (Figs. 1B & C). C-Reactive Protein (CRP) was 28.43 mg/dL, and White Blood Cell count (WBC) was 11,300 /mm3. RT-PCR for SARS-CoV-2 was positive. He was intubated and ventilated. Favipiravir (3,200 mg/day, 1,600 mg b.i.d.), chloroquine (200 mg/day, s.i.d.), and antibiotics (ceftriaxone sodium hydrate 2 g/day, azithromycin hydrate 500 mg/day) for bacterial infection were started. On April 15, the patient’s SpO2 was 99% with 0.5 inspired oxygen fraction (FiO2). His CRP was 19.99 mg/dL, and WBC was 7600 /mm3. 5-ALA (1500 mg/day, 500 mg t.i.d.) with SFC was administered through a nasogastric tube, and ciclesonide (400 μg/day: 200 μg b.i.d.) was given. The dose of favipiravir was reduced to 1,200 mg/day and that of chloroquine was increased to 400 mg/day. On the next day (April 16), his SpO2 recovered to 97.3% with 0.25 FiO2. His CRP was 21.65 mg/dL, and WBC was 8,700 /mm3. On April 17, his clinical symptoms improved, and favipiravir and chloroquine were completely discontinued due to high AST (49 IU/L) and ALT (61 IU/L) values. However, 5-ALA with SFC and ciclesonide were continued. On April 18, he was disconnected from the ventilator, when his SpO2 reached 93.6% with 1 L/min nasal oxygen. The patient’s CRP was 23.99 mg/dL, and WBC was 8,600 /mm3. On the same day, 5-ALA was reduced to 750 mg/day (250 mg t.i.d.). On April 19, the patient was transferred from the ICU to the general ward. His symptoms further improved, and he was free from nasal oxygen on April 22. His SpO2 was 90% on room air, and his CRP decreased to 1.50 mg/dL. Chest X-P on April 23 showed improvement of pneumonia of the right upper lobe (Fig. 1D). On April 24, the liver enzyme levels in blood such as AST and ALT decreased to normal levels. RT-PCR was carried out on April 24 and 26. The results of both RT-PCRs were negative, and he was discharged. He is now living a normal life with no complications or sequelae. The serum anti-SARC-CoV-2 IgM and IgG antibody levels were too low to be detected by a conventional qualitative assay.
3.2. Case 2
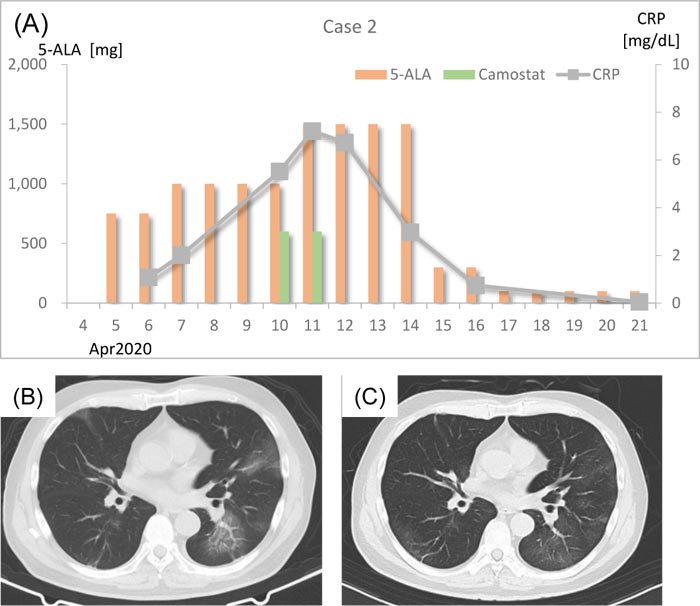
In the beginning of April 2020, a 69-year-old man experienced common cold–like symptoms. On April 5, he visited the clinic because his wife was diagnosed with COVID-19 by RT-PCR. He was suspected to have SARS- CoV-2 infection, and 750 mg/day (250 mg t.i.d.) 5-ALA with SFC was prescribed (Fig. 2A). Chest CT scan on April 7 showed bilateral viral pneumonia shadows with ground-glass opacities extending to the subpleural area (Fig. 2B). His body temperature was 37.1°C, SpO2 was 96% on room air, and CRP was 2.01 mg/dL. The dose of 5-ALA was increased to 1,000 mg/day (250 mg q.i.d.). On April 10, the patient’s body temperature was 37.5°C and his CRP was elevated to 5.52 mg/dL. Camostat mesilate (600 mg/day, 200 mg t.i.d.) was added together with antibiotics (tetracycline hydrochloride 100 mg /day, ampicillin sodium 2 g/day, sulbactam sodium 1g/day) against bacteria. Antibiotics were prescribed for 3 days. On April 11, the patient’s body temperature was 36.9°C. However, his CRP had further increased to 7.21 mg/dL, and the 5-ALA dose was increased to 1,500 mg/day (500 mg t.i.d.). On April 12, his CRP decreased to 6.73 mg/dL. Clinical symptoms were improved on April 14, and his fever had subsided. The patient’s CRP decreased to 2.97 mg/dL as well. Beginning on April 15, the dose of 5-ALA was reduced to 300 mg/day (100 mg t.i.d.). Antibodies against SARS-CoV-2 were examined on April 16. Both IgM and IgG were positive. From April 17, the dose of 5-ALA was further decreased to 100 mg/day (50 mg b.i.d.). A chest CT scan was performed on April 21, which revealed a partial improvement of pneumonia, and the patient’s CRP had achieved a normal level (0.05 mg/dL). Chest CT scan taken on May 7 revealed almost complete remission of pneumonia (Fig. 2C). He is now living a normal life with no complications or sequelae. The last RT-PCR was negative.
3.3. Case 3
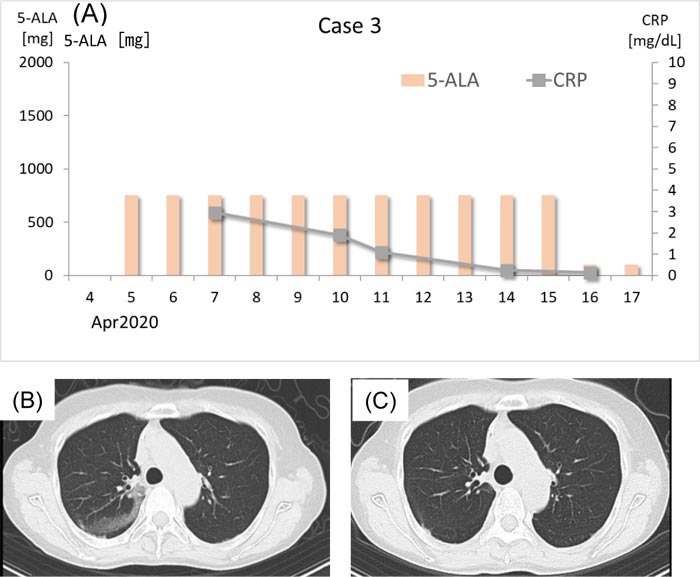
A 66-year-old woman became aware of a slight fever on March 31, 2020. A few days prior, she had dinner with an intimate friend who was later diagnosed with COVID-19. On April 5, the patient experienced a sore throat and visited the clinic. She used to be a smoker (40 cigarettes/day for 38 years) but had stopped smoking for the past 9 years. She was suspected to have SARS-CoV-2 infection, and 750 mg/day (250 mg t.i.d.) 5-ALA with SFC was prescribed (Fig. 3A). On April 7, a chest CT scan was performed, which revealed ground-glass opacity in the right upper lobe (Fig. 3B). Her CRP level was 2.95 mg/dL. Thereafter, her CRP gradually decreased, and it was 0.13 mg/dL. When antibodies against SARS-CoV-2 were examined on April 16, both IgM and IgG were positive. On the same day, the 5-ALA dose was reduced to 100 mg/day (50 mg b.i.d.). Chest CT scan on April 21 revealed almost complete resolution of the initial pneumonia, although a new subtle lesion was detected in the subpleural area of the left lower lobe. Her CRP level was 0.05 mg/dL when she visited the clinic on May 2. CT scan on May 7 revealed almost complete resolution of the pneumonia shadow (Fig. 3C). She has been afebrile with normal SpO2 throughout the clinical course. She is now living a normal life with no complications or sequelae. The last RT-PCR was negative.
3.4. Case 4
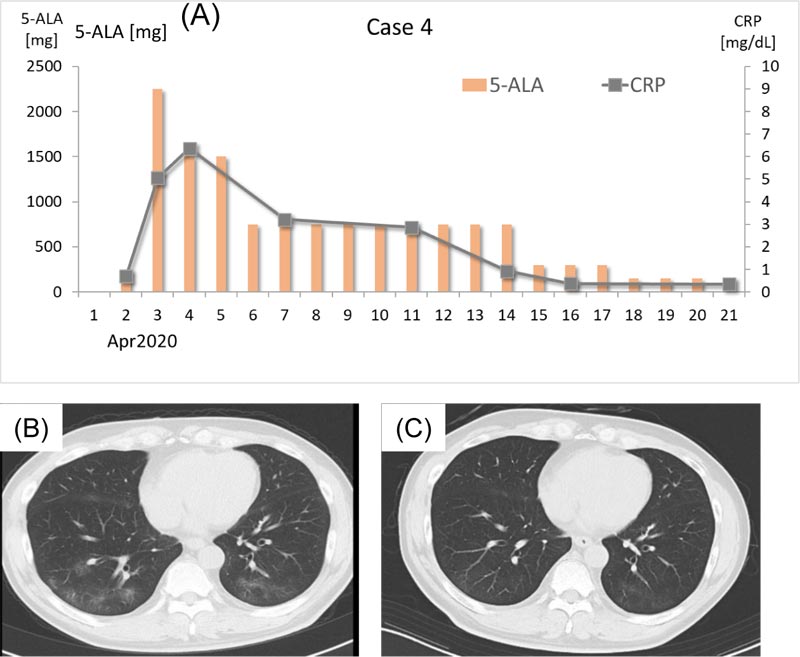
A 63-year-old man felt a chill and visited the clinic on April 2, 2020. He had been experiencing common cold–like symptoms for a few days prior to clinic presentation. He had been a smoker until 2 years previously (10 cigarettes/day for 30 years and 5 cigars/day for 5 years) but had stopped smoking for the past 2 years. At the time of his visit, his body temperature was 36.3°C, and his CRP level was 0.70 mg/dL. Antibiotic therapy (tetracycline hydrochloride 100 mg/day, ampicillin sodium 2 g/day, sulbactam sodium 1 g/day for the initial 2 days) and 250 mg/day 5-ALA (125 mg b.i.d.) with SFC were prescribed (Fig. 4A). On the next day (April 3), the patient’s fever increased to 38.5°C and the CRP level was elevated to 5.06 mg/dL. Chest CT scan revealed bilateral multiple nodular shadows with ground-glass opacity lesions (Fig. 4B). That day, the dose of 5-ALA was increased to 2250 mg/day (750 mg before dawn and 500 mg t.i.d. during daytime), and the dose was decreased to 1,500 mg/day (500 mg t.i.d.) on April 4. On April 6, the patient experienced watery diarrhea, and 5-ALA was reduced to 750 mg/day (250 mg t.i.d.). Beginning on April 7, the patient’s body temperature decreased to below 37.0°C, and his CRP gradually decreased. Antibodies against SARS-CoV-2 were examined on April 16. Both IgM and IgG were positive. The dose of 5-ALA was decreased to 300 mg/day (100 mg t.i.d.) on April 15, and it was further decreased to 150 mg/day (50 mg t.i.d.) on April 18. Chest CT scan examined on April 21 did not show a distinct improvement, but his CRP level had decreased to 0.34 mg/dL. However, a chest CT scan performed on May 7 revealed almost complete resolution of the pneumonia shadow (Fig. 4C). He is now living a normal life with no complications or sequelae. The last RT-PCR showed negative.
3.5. Case 5
A 67-year-old man visited the clinic on April 10, 2020, because he had a slight fever (about 37.5°C) for 1 week. At the time of visit, his body temperature was 36.1°C, CRP level was 4.92 mg/dL, and SpO2 was 97% on room air (Fig. 5A). Chest CT scan on the same day revealed multiple lesions with ground-glass opacity in the subpleural area (Fig. 5B). A dose of 750 mg/day 5-ALA (250 mg t.i.d.) with SFC and camostat mesilate (600 mg/day, 200 mg t.i.d.) were prescribed. The dose of 5-ALA was increased to 1,500 mg/day (500 mg t.i.d.) and camostat mesilate was terminated on April 12. The patient’s CRP level decreased to 0.93 mg/dl on April 14, and 5-ALA was reduced to 750 mg/day (250 mg t.i.d.) on April 15. On April 16, antibodies against SARS-CoV-2 were examined. IgM was negative, but IgG was positive. The CRP level was 0.27 mg/dL, and 5-ALA was reduced to 100 mg/day (50 mg b.i.d.). Chest CT scan on April 21 revealed considerable improvement of lung lesions, and the patient’s CRP had further decreased to 0.08 mg/dL. Chest CT scan on May 7 revealed almost complete resolution of the pneumonia shadow (Fig. 5C). He returned to normal life without problems. The last RT-PCR was negative.

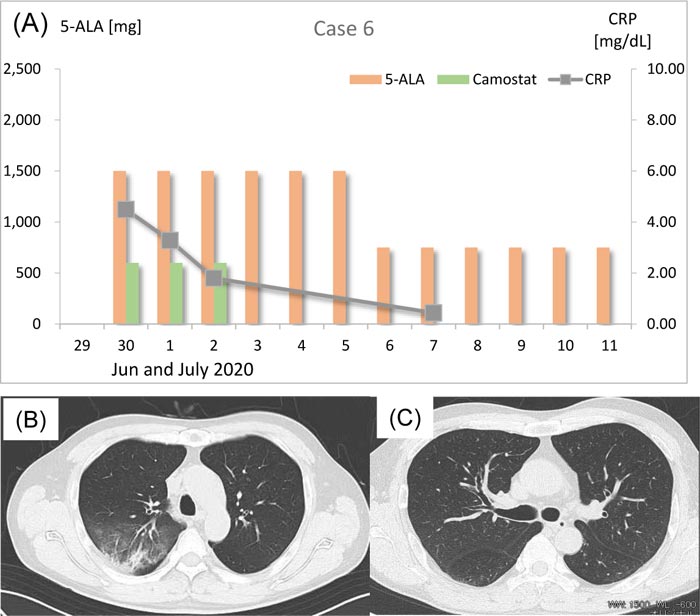
3.6. Case 6
On June 26, 2020, a 56-year-old man experienced common cold–like symptoms with high fever. His body temperature was 37.5 to 38.5 °C. He took OTC antifebrile pills and got his body temperature down to 36.5 °C. However, he became suspicious about his infection by SARS-CoV-2 because he lost taste and smell, and then he visited the clinic. His CRP was 4.49 mg/dL and RT-PCR test showed positive for SARS-CoV-2. On June 30, his chest CT scan showed bilateral viral pneumonia shadows with ground-glass opacity in the right upper lobe (Fig. 6B). Thus, 1,500 mg/day (500 mg t.i.d.) 5-ALA with SFC was prescribed (Fig. 6A). He was also treated with 600 mg/day camostat mesilate (200 mg t.i.d.) together with antibiotics (tetracycline hydrochloride 100 mg/day, ampicillin sodium 2 g/day, sulbactam sodium 1 g/day for the initial 3 days) against bacterial infection. On July 1, he was given the same treatment and his body temperature was 36.8°C, SpO2 was 97% on room air, and CRP was lowered to 3.28 mg/dL. On July 2, he was hospitalized and given 1,500 mg/day (500 mg t.i.d.) 5-ALA with SFC and 600 mg/day camostat mesilate (200 mg t.i.d.) again.
His CRP level decreased to 1.80 mg/dL. He was given only 1,500 mg/day (500 mg t.i.d.) 5-ALA with SFC for 3 more days without any other medications. During his hospitalization, he had a high fever (38.0°C) only once in the night on June 3 but recovered soon by antipyretics. On July 7, the patient’s body temperature was 36.6°C and his CRP decreased to 0.44 mg/dL, SpO2 was 98% on room air, and then he was dismissed. The dose of 5-ALA was decreased to 750 mg/day (250 mg t.i.d.) on July 6 and the same treatment was given at home until July 12 for total 6 days.
On July 14 he visited the clinic. Both IgM and IgG for SARS-CoV-2 were positive and his chest CT scan revealed almost complete remission of pneumonia (Fig. 6C). He is now living a normal life with no complications or sequelae. The last RT-PCR was negative.
3.7. Side Effects
High doses of 5-ALA with SFC did not cause serious side effects except for a few cases as follows. In the cases 2, 4, and 5, the patients experienced abdominal swelling, constipation and had blackish feces when 1,500 mg/day 5-ALA with SFC was administered. In case 2, he had skin irritation by sun light exposure after taking 1,500 mg/day 5-ALA with SFC. A patient who had arrhythmia reported palpitations when he took 1,500 mg/day 5-ALA with SFC. Those side effects were all dissolved by dose reduction to 750 mg/day.
Other minor side effects and complaints were abdominal pain, diarrhea, nausea, glow, malaise, and insomnia. None of them were serious and all of them were resolved after the dose reduction.
During the treatment of COVID-19 patients with 5-ALA with SFC, blood liver enzyme levels such as AST (aspartate aminotransferase, GOT)and ALT (alanine aminotransferase, GPT) were normal in all the cases except for the initial period of Case 1. When Case 1 patient was treated with favipiravir and chloroquine, both AST and ALT exceeded the normal ranges as described above. However, after the termination of dosing of favipiravir and chloroquine, both AST and ALT levels decreased to normal levels.
4. DISCUSSION
The present cases demonstrate early recovery from COVID-19 after the administration of 5-ALA with or without other candidate drug(s). The mean length of hospitalization of COVID-19 patients is shortened from 15 days to 11 days with remdesivir [27] and it is from 15 days to 14 days with favipiravir [28]. In the present cases 1, 2, 3, 5, and 6, a prominent improvement was observed within 7-10 days accompanied by normalization of CRP level, whereas it took nearly 2 weeks in case 4, who was a heavy smoker. There were no serious side effects of the applied supplements. Only minor problems such as abdominal swelling, constipation, blackish feces, and skin irritation were reported, and all dissolved by dose reduction of 5-ALA and SFC. Abdominal swelling, constipation, and blackish feces are known as side effects of SFC (Ferromia®) approved for the treatment of anemia and iron deficiency, and all of them can be improved by dose reduction. Skin irritation has been reported as a rare side effect of 5-ALA hydrochloride for fluorescence guided surgery of high-grade glioma (Gliolan®, Gleolan®, and Alabel®) [29].
Because the mean length of hospitalization of COVID-19 patients in Japan has been reported to be 16.6 days [30], it appears that the drugs used in the present case shortened the duration of treatment. In cases 3 and 4, 5-ALA with SFC alone effectively caused early recovery. When these results are taken together with the results of other cases, 5-ALA appears to be efficacious for COVID-19 treatment.
Deadly viral infection often causes a cytokine storm and can damage the blood vascular structure in various organs [31]. This process is associated with tissue inflammation, in which resident inflammatory cells and immune functional cells are activated and release a variety of inflammatory cytokines, leading to serious vascular damage and an increase in blood vessel permeability [31].
To stop the overreaction of inflammatory cells, adequate induction of regulatory cells is required. Reactive oxygen species should also be extinguished in such inflammatory tissues. Interestingly, HO-1 is a perfect key molecule in the body for achieving these complicated tasks [12]. Macrophage polarization to regulatory status is induced by HO-1, and the immune tolerance mediated by regulatory dendritic cells and regulatory T cells is also performed through HO-1 induction [32, 33]. The supplement of 5-ALA with SFC has been shown to efficiently induce HO-1 expression and enhance HO-1 activity in peripheral blood monocytes as well as in tissues of various organs [15, 16]. Oral administration of 5-ALA alone did not induce HO-1 in peripheral blood mononuclear cells, while 5-ALA with SFC did [15, 16]. Therefore, we employed 5-ALA with SFC in the present clinical research.
HO-1–catalyzed degradation products of heme are biliverdin, CO, and ferrous ions, and biliverdin is further converted by biliverdin reductase to bilirubin, the strongest antioxidant in our body. All heme metabolites are potent reducing agents and a potential role of the antioxidant action of bilirubin has widely been discussed in various therapeutic areas including its activity to prevent oxidative stress-induced acute cholestasis [34]. In addition, biliverdin has been reported to exert antiviral activity via its binding to RNA viral proteases [35], and it has emerged as a cytoprotective and important anti-inflammatory molecule [36].
Recently, protoporphyrin IX (PPIX) has been reported as a potent inhibitor of SARS-CoV-2 infection and replication in vitro [37]. PPIX binds to both ACE2, a cellular ligand for SARS-CoV-2 virus, and cell membrane lipids, whereby it inhibits coronaviral infection on those cells [37, 38]. PPIX has shown much stronger efficacy for SARS-CoV-2 infection compared with remdesivir, an ebola virus RNA polymerase inhibitor whose therapeutic application for COVID-19 was urgently approved by the Food and Drug Administration [37]. It is well known that the oral uptake of high-dose 5-ALA leads to the production and accumulation of PPIX in tumors and inflammatory tissues [39]. Therefore, it is plausible that not only heme and HO-1 but also PPIX are produced in the virus-infected inflammatory tissues and that those molecules synergistically suppress both inflammation and virus replication.
Furthermore, guanine quadraplex (G4) structures in the RNA genome of SARS-CoV have been speculated to play an important role in the regulation of viral replication [40]. We have identified 25 putative G4 structures in the SARS-CoV-2 genome (data not shown). Heme and PPIX are potent ligands for G4 structure and could modulate G4 function not only in mammalian cellular and mitochondrial gene replication and expression but also in RNA viral replication and transcription [41-44]. Taken together, these results allow us to speculate on the significant involvement of heme and PPIX in the replication and infection of SARS-CoV-2. Therefore, not only HO-1 induction but also the production of heme and PPIX are considered to orchestrate the prevention of disease progression of COVID-19 and enhance the recovery of COVID-19 patients. In fact, a group of virologists in Institute of Tropical Medicine (NEKKEN), Nagasaki University, reported the potent inhibition of infection and growth of SARS-CoV-2 in vitro by using green monkey VeroE6 cells and human Caco-2 cells as host cells [45]. Then Nagasaki University has very recently announced the initiation of clinical research for the treatment of COVID-19 patients with 5-ALA in collaboration with Neopharma Japan [46]. In addition, Royal College of Surgeon in Ireland, Medical School of Bahrain and Bahrain Defense Force Hospital have registered their clinical trials on COVID-19 with 5-ALA and SFC to NIH, USA [47].
Possible side effects of 5-ALA hydrochloride are listed in the drug package insert of Gliolan® for photodynamic diagnosis of glioma as well as Alaglio® for bladder cancer (mainly liver dysfunction, nausea and vomiting). In addition, 5-ALA phosphate plus SFC has been marketed as an approved supplement in more than 7 countries including Japan, UAE, Bahrain, and Jordan. Adverse events caused by high doses of 5-ALA with SFC were mainly mild gastrointestinal symptoms, while significant photosensitivity as well as skin damage were not observed [20]. As explained in the introduction, a supplement formulation of 5-ALA with SFC has been marketed for more than 10 years in Japan. It has been registered as a food with functional claims for people with prediabetes or sleep problems because the active ingredient of these supplements, 5-ALA phosphate, is a fermentation product with enough safety profiles required for food supplements. The results from the present case study also support the tolerability and safety of 5-ALA with SFC in the MTD.
In the clinical cases described in this report, the hepatic functions were monitored. It is necessary to monitor the levels of ALT and AST considering the possibility of viral hepatitis and hepatotoxicity induced by antiviral drugs such as favipiravir and remdesivir that have been used for the therapy of COVID-19 patients and known to cause severe hepatotoxicity [48, 49]. The amount of 5-ALA for oral administration should be increased or decreased in a timely manner based on the inflammatory reaction, the numbers of blood cells, the degree of fever, the degree of pneumonia, the oxygen concentration, the body weight, and hepatic conditions as well.
CONCLUSION
In conclusion, HO-1 induction by 5-ALA with SFC could rescue COVID-19 patients in the acute phase and promote their recovery process. This study revealed no significant adverse events after multiple administrations of 5-ALA with SFC at the MTD. Therefore, a clinical trial of 5-ALA with SFC in COVID-19 patients might be urgently needed to allow the delivery of this safe therapeutic supplement to COVID-19 patients considering the recent rapid expansion of the third global pandemic of COVID-19.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The ethical approval for this study was obtained from The Ethical Committee of Hanzomon Gastrointestinal Clinic on April 1st, 2020.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written and informed consent from the patients were obtained for publishing this case report.
AVAILABILITY OF DATA AND MATERIALS
The clinical data available under the agreement with the patients were all shown in the report. If necessary, other data will be provided upon receiving an appropriate request and obtaining further agreement from the individual patient.
FUNDING
There is no financial support or public/private grant for the case studies.
CONFLICT OF INTEREST
Dr. Kazutoshi Kaketani has no conflict of interest. Dr. Motowo Nakajima is a management board member of SBI Pharmaceuticals Co., Ltd.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Namiki Izumi, Japanese Red Cross Musashino Hospital, Tokyo 180-8610, Japan, for his kind contribution to this clinical research. The authors also greatly appreciate Drs. Naohide Yamashita and Kiyotaka Fujine (Neopharma Japan) for their excellent assistances in preparing this manuscript.
