All published articles of this journal are available on ScienceDirect.
SARS-CoV-2 Infection and Transmission Inhibitor
Abstract
As of December 2019, there has been an outbreak of severe respiratory syndrome coronavirus-2 (SARS-CoV-2) in China. Around 16% of patients developed acute respiratory distress syndrome, and 1%–2% died. There is no specific treatment reported. A pandemic related to Coronavirus is one of the most significant health crises in human history. In spite of a massive influx of cases, SARS-CoV-2 is still untreatable. Both old and novel virologic medicines were tested in order to develop a novel drug or vaccine. In advanced clinical trials in the US, favipiravir is currently approved for treating influenza infections in Japan, and it has been approved for treating SARS-CoV-2 or COVID-19 infections in Japan. Studies have shown that favipiravir can treat mild to moderate COVID-19 infections. The present review examines the role and use of favipiravir in the treatment of COVID-19. The purpose of this review is to provide evidence obtained from a variety of authentic sources. Current research is evaluating favipiravir for treating Coronavirus infections.
1. INTRODUCTION
COVID-19 consists of long polymers bounded by nucleocapsids, and it spreads through a variety of routes, including aerosolized transmission, fecal-oral transmission, droplets, and surface transmission. The disease affects multiple organs including the lungs, heart, and kidneys. There is a possibility of respiratory failure, pneumonia, or even death due to infections. It is common to suffer from acute lung and renal injuries and shock. The virus is detected by PCR testing from nasopharyngeal swabs. The virus and illness that Covid-19 causes are unknown, and there are no treatments available. There is no specific treatment available without symptomatic treatment [1].
The drug favipiravir is used to stop SARS-CoV-2 signs. A spike in research occurred after COVID-19 broke out to find a cure or medication. In order to combat COVID-19 (Coronavirus disease), triggered by the SARS-CoV-2 virus, a variety of drugs may be used, including favipiravir and others [2]. The drug favipiravir obstructs RNA-dependent RNA polymerase (RdRp) in RNA viruses in a broad-based and potent manner. Since January 12, 2021, more than 88 million cases along with 1.9 million mortality cases have been reported worldwide [3]. Despite the difficulty of predicting the threshold [4], SARS-CoV-2 spread is unlikely to stop until at least 50% of the population is immune, either through vaccination or after recovering from a naturally acquired infection [5]. There are now some promising vaccine candidates approved by the FDA [6] in an emergency situation. It is, however, anticipated that global access to the necessary level will be delayed until the pandemic reaches the population impact level.
As shown in Table 1, Coronavirus causes disease in humans and their cellular receptors.
The covid-19 pandemic has led to few effective treatments being developed to cope with this disease. Initially, favipiravir was applied to SARS-CoV-2 in Wuhan, where the epicenter of the pandemic was located. Italian authorities approved the prescription of the drug for emergency purposes after the pandemic spread to Europe. The drug is currently being marketed in Japan, Russia, Ukraine, Uzbekistan, Moldova, and Kazakhstan. Regions like Saudi Arabia and the United Arab Emirates have also delivered the respective approval to the said drug. Afterward, Turkey, Bangladesh, and most recently Egypt launched this agent for commercial use category. In the year 2020 during the month of June approval of favipiravir was received for mild and moderate cases of COVID-19 infections in India from the DCGI. On Clinical Trials Gov, there were 32 studies registered as of the 23rd of July, 2020; three are complete, and 12 (twelve) are recruiting [7].
According to the Japanese Pharmaceuticals and Medical Devices Agency, favipiravir (AVIGAN®) was approved for the treatment of acute and re-emerging influenza virus infections in 2014 [8]. The majority of studies have reported its effectiveness against other RNA viruses, such as Ebola viruses [8, 9] or rhinoviruses and respiratory syncytial viruses [10]. The 50% effective concentration (EC50) of favipiravir against SARS-CoV-2 was reported to be 61.88 μM/L in Vero E6 cells in vitro studies. Therefore, favipiravir is highly effective at treating COVID-19 patients. Despite this, there is very limited research examining the safety as well as efficacy aspects of favipiravir in COVID-19 patients.
The viral inhibitory activity of favipiravir (T-705) was first identified during the study of chemical agents that can neutralize influenza viruses in the chemical library of Toyoma chemicals [11]. There were antiviral activities linked with the first compound, A/PR/8/34, which was later, named asT-1105, and it’s their associated derivatives. The pyrazine moiety in the structure of T-1105(2) was chemically modified to yield the drug favipiravir.
| Group | Virus | Host | Disease Caused | Cellular Receptor |
|---|---|---|---|---|
| I | 229E | Human | An infection of the respiratory system | Human APN |
| NL-63 | Human | An infection of the respiratory system | ACE-2 | |
| II | OC43 | Human | Enteric and respiratory infections | Neu-5,9-Ac-2-containing moiety |
| HKU1 | Human | Asthma | - | |
| SARS-C0V | Human | SARS (severe acute respiratory syndrome) | ACE-2 |
2. STRUCTURE
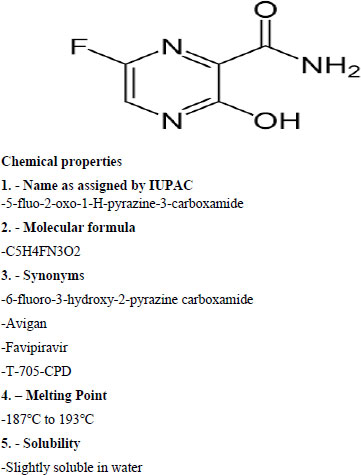 |
3. PHARMACOKINETICS AND PHARMACO- DYNAMICS
According to studies conducted on healthy Japanese volunteers, the maximum plasma concentration of favipiravir occurred 2 hours after oral administration, and then the concentration decreased rapidly with a half-life of 2 to 5.5 hours [12]. Favipiravir is administered as a prodrug. In addition to its excellent bioavailability (>94%), it is 52 percent protein bound and has a low volume of distribution (10–20 liters). It reaches C-max within 2 hours after a single dose. As a result of multiple doses, both T-max and half-life increase. Due to the short half-life (2.5-5 h) of favipiravir, its hydroxylated form is rapidly eliminated through the kidneys. In addition to aldehyde oxidase, xanthine oxidase plays a marginal role in the elimination process. The pharmacokinetics of favipiravir is dose-dependent and time-dependent. The compound is not metabolized by cytochrome P-450, but it inhibits one of its components (CYP2C8). The medication should be used with caution when combined with drugs that are converted by CYP2C8 [13, 14]. Fig. (1) illustrates Favipiravir's intracellular activation.
4. ASSESSMENT METHOD
Favipiravir has a novel mechanism of action in comparison to existing influenza antivirals that prevent the virus from entering and exiting cells [14]. As a result of its selective RNA inhibition properties, favipiravir-RTP inhibits RNA when activated. The polymerase prevents replication of the viral genome [15]. There is evidence that favipiravir-RTP interacts with RNA-dependent RNA polymerase (RdRp) under multiple hypotheses [16]. In spite of the fact that favipiravir was originally intended to treat influenza, the RdRp catalytic domain (favipiravir primary target) is expected to have similarities to other RNA viruses [9]. When the drug is administered into the body, it undergoes phosphoribosylation to become favipiravir-RTP. The following mechanisms are responsible for its antiviral effect:
A - This molecule serves as a substrate for RNA-dependent RNA polymerase (RdRp), which mistakes it for a purine nucleotide [7], thus inhibiting its activity.
B - The RNA strand is incorporated into the viral strand, preventing further expansion of the virus. Several RNA viruses retain the catalytic domain of the RdRp enzyme, allowing this drug to have a broad spectrum of activity.
According to recent findings, favipiravir causes lethal mutations during influenza virus infection in vitro [17]. Similar activity has not yet been demonstrated against SARS-CoV-2. Figs. (1 and 2) show Favipiravir's overall mechanism of action in its active and inactive forms, as well as how it interacts with the body's cell surface. In Fig. (2), we see a more detailed demonstration of how the drug interacts with living organisms.
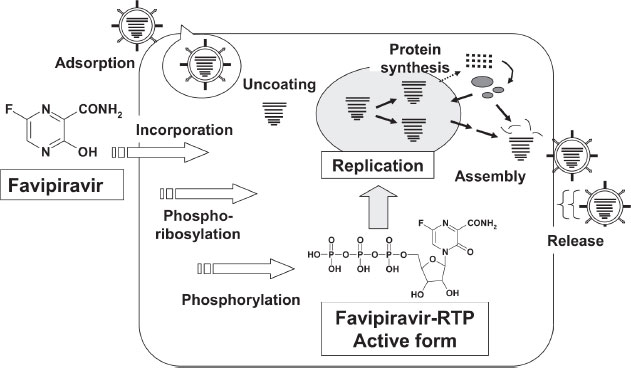
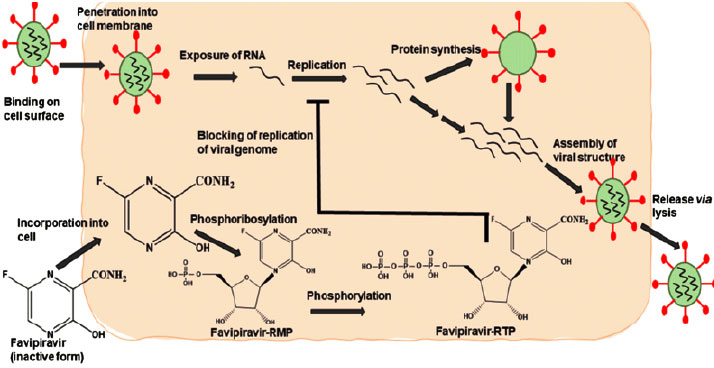
5. THE PHARMACOLOGICAL IMPLICATIONS
There are several RNA viruses that are resistant to favipiravir.
5.1. Influenza
The favipiravir molecule was discovered by chemically modifying pyrazine analogs in cells that exhibited anti-influenza virus activity [18]. There is an antiviral medicine called FAVIPIRAVIR. As a selective and powerful inhibitor of influenza viral RNA polymerase, the drug works well against many influenza virus strains and subtypes. A study found that favipiravir was also effective against influenza viruses resistant to neuraminidase inhibitors, which included oseltamivir [19].
5.2. COVID-19
Several clinical trials with favipiravir are currently being conducted to assess its efficacy and safety against SARS-CoV-2 [20]. Several countries, including India, have now approved the drug. According to DCGI, this drug is approved for use in cases of mild to moderate severity in India. The medication can only be prescribed once the patient or their representative has given informed consent for emergency use. During Day 1, it is recommended to take 1800 mg or 9 tablets of 200 mg twice a day. Then, for a maximum of 14 days, you can take 800 mg or four 200 mg tablets twice daily.
5.3. Ebola
In 2014, the World Health Organization shortlisted favipiravir for trial during the Ebola outbreak. Clinical studies have indicated that this drug may enhance survival, based on in vitro results [21]. Insufficient evidence was found to support the claim of benefit.
5.4. Clinical Trials
Over the past few months, clinical studies have been performed all over the world to assess the efficacy of favipiravir in the management of COVID-19. The major clinical studies are summarized here [22]. According to Table 2, favipiravir exhibits a number of pharmacological indications backed by scientific evidence and clinical trials.
| Study Type | Trial Outcome and Design | Conclusion |
|---|---|---|
| Exploratory Randomized, Controlled Trial | 29 confirmed cases of COVID-19 were randomized (1:1:1) to Favipiravir for 14 days, Baloxavir-Marboxil (80 mg once daily orally on Days 1 and 4), or a control group. Lopinavir/ritonavir (400 mg/100 mg, bid, orally) and darunavir/cobicistat (800 mg/150 mg, qd, orally) as well as arbidol (200 mg, tid, PO) were administered to all patients. Interferon was required in all of them. The PCR levels were undetectable in all control groups on day 14 and 77% and 70% respectively in the Baloxavir and Favipiravir groups. Moreover, clinical improvements were similar between all groups. The baloxavir-marboxil and favipiravir groups both had one patient transferred to the ICU within seven days of the start of the trial. Among the 29 patients, there were no deaths. |
The study results fail to support the hypothesis that adding baloxavir or favipiravir to COVID-19 patients' existing standard treatment would benefit them [22]. |
| Trial of prospective, randomized, controlled, open-label nature. | The study randomized 236 moderate/severe COVID-19 cases: 116 received Favipiravir for 10 days, and 120 took Umifenovir (Arbidol) for 10 days. All patients received conventional treatment. Clinical improvements at day 7 (the primary endpoint) were not significant between groups. Despite this, posthoc analysis for moderate COVID-19 patients showed significant enhancement in the Arbidol group (62/111, 55.86%) than in the Favipiravir group (70/98, 71.43%). Both pyrexia and cough were relieved faster with favipiravir. The rate of auxiliary oxygen therapy (AOT) and non-invasive mechanical ventilation (NVM) did not differ significantly between the groups. |
A comparison of Favipiravir and Arbidol did not significantly improve clinical recovery rates. Pyrexia and cough relief were significantly improved by favipiravir [22]. |
| Control study with open-label.35 | For 14 days, 80 mild to moderate severity cases of COVID-19 were assigned to either Favipiravir (n = 35) or Lopinavir / Ritonavir (n = 55) (control group). In addition, both groups received aerosolized interferon (IFN). It was seen that favipiravir led to improved outcomes compared to the control arm, such as a shorter viral clearance period of 4 (2.5–9) days vs 11 (8–13) days, P = 0.001, and significant improvements in chest imaging (91.43% vs 62.22%, P = 0.004). | COVID-19 was significantly better treated with favipiravir in terms of disease progression and viral clearance [22]. |
| Involvement-oriented, randomized, open-label phase 3 trial | In this study, 100 patients with COVID-19 were recruited. After receiving 3200 mg of favipiravir on day 1, the patients received 600 mg twice (days two through ten). In 50 patients, hydroxychloroquine was administered at 800 mg on day 1, 200 mg twice (days 2–10), and oral oseltamivir at 75 mg/12 h/day for ten days. A significant difference between the HCQ arm and the favipiravir arm was not observed in terms of the detection of SARS-CoV-2 by PCR (p = 0.7). However, favipiravir more frequently induced fevers and dry coughs (24% and 30%, respectively) than HCQ-based therapy (p > 0.05). Favipiravir caused elevated liver transaminases in 4 patients (8%) between Days 7 and 14, but they improved within two weeks after stopping the drug. As a result of myocarditis, one person died on Day 8 in the HCQ-based arm. | - |
| - | ≥ 1% | 0.5- ˂ 1% | ˂ 0.5% |
|---|---|---|---|
| Hypersensitivity | - | Resh | Eczema, Proteus |
| Hepatic |
AST (GOT) increased
A/T (GPT) increased γ GTP increased |
- | Blood ALP increased, blood half-life increased |
| Gastrointestinal | Dantoe | Nausea, vomiting abdominal pain | Abdominal discomfort, ulcer, haematochatis, goiter |
| Hematologic | Neutrophil count decreased, white blood cell count decreased | Glucose urine present | White blood cell count increased, reticulocyte count decreased, monocyte increased |
| Metabolic disorder | Uric acid increased, and Blood triglyceride increased | - | Blood potassium decreased |
| Respiratory | - | - | Asthma, oropharyngeal pain, rhinitis, nasopharyngitis |
| Others | - | - | CPK increased blood urine present, tonsil, vision blurred, eye pain, vertigo |
6. IMPACTS OF ADVERSE EVENTS
Despite its adverse effects, favipiravir is generally considered safe, as confirmed by a large systematic review [23]. Here are some brief details about this drug's adverse effects. It is associated with an increase in total uric acid in the blood, diarrhea, a decreased white blood cell count (neutrophils), higher liver enzyme levels, and higher triglyceride levels in the blood.
- Symptoms of diarrhea
- Elevated uric acid levels (hyperuricemia).
- Neutrophil count reduced
- Having nausea
- Ingestion of vomit
- Pain in the abdomen
We are all aware of the risks associated with each drug. However, any drug should be used only if it has a minimal number of adverse effects and a high therapeutic effect. According to Table 3 , some adverse effects of favipiravir are minor, negligible, or less severe. Based on a large systematic review, this drug is effective in treating COVID-19 and safe in treating antiviral infections.
CONCLUSION
The COVID-19 infection is unlikely to disappear in the near future. Multiple clinical trials have been conducted on favipiravir against the devastating SARS-CoV-2 virus because of its novel antiviral activity and broad spectrum of activity. A recent study suggests that favipiravir can minimize viral clearance time and improve chest symptoms. The drug is considered relatively safe when used for a short period of time. Aside from being administered orally, in symptomatic patients not sick enough to require hospitalization, favipiravir is preferred. Due to the fact that most COVID-19 patients (85%) have mild to moderate disease and can be treated at home, this drug could potentially be beneficial for many patients. Additionally, favipiravir is being studied in combination with umifenovir to see if they complement one another or act synergistically [22, 23]. As for the side effects of the drug, asymptomatic hyperuricemia and slight, reversible elevations in transaminases are reported most frequently. Consequently, Favipiravir appears to be effective in treating mild to moderate symptoms associated with SARS-CoV-2 infection. A comprehensive review of the antiviral research conducted over the course of the last decade describes how favipiravir has shown to be experimentally useful against COVID-19 and describes how it has been compared to other antiviral drugs. It is possible to clarify the antiviral properties of this drug using bioinformatics databases and tools. Clinical and experimental studies have demonstrated that this drug can cure COVID-19 disease.
LIST OF ABBREVIATIONS
| µM/L | = Micromole |
| AAS | = Antiphospholipid Antibody Syndrome |
| ACE | = Angiotensin-converting Enzyme |
| AOT | = Auxiliary Oxygen Therapy |
| APN | = Access Point Name |
| BBB | = Blood Brain Barrier |
| CDC | = Centre For Disease Control |
| CNS | = Central Nervous System |
| COVID-19 | = Corona Virus Disease 19 |
| CYP-2C-8 | = cytochrome P450 family 2 subfamily C member 8 |
| DCGI | = Drug Controller General of India |
| DPPH | = 2,2-Diphenyl -1 Picrylhydrazyl |
| EC-50 | = Effective Concentration |
| FDA | = Food & Drug Administration |
| HCQ | = Hydroxychloroquine |
| HIV | = Human Immunodeficiency Virus |
| ICU | = Intensive Care Unit |
| IFNs | = Interferons |
| IL | = Interleukin |
| IMD | = Immunological Diseases |
| IUPAC | = International Union of Pure and Applied Chemistry |
| LDL | = Low-Density Lipoprotein |
| LPS | = Lipopolysaccharide |
| NIH | = National Institute of Health |
| NVM | = Non-invasive Mechanical Ventilation |
| PCR | = Polymerase Chain Reaction |
| PCT | = Porphyria Cutanea Tarda |
| PDCs | = Plasmacytoid Dendritic Cells |
| PLE | = Polymorphic Light Eruption |
| RA | = Rheumatoid Arthritis |
| RdRp | = RNA-dependent RNA polymerase |
| RNA | = Ribonucleic Acid |
| RTP | = Real-time Transport Protocol |
| SARS-CoV-2 | = Severe Acute Respiratory Syndrome- Corona Virus 2 |
| UAE | = United Arab Emirates |
| US | = United States of America |
| USCDC | = United States Centers for Disease Control and Prevention |
| WHO | = World Health Organization |
AUTHOR CONTRIBUTIONS
The review is outlined by Subhash Chandra and Sakshi. The manuscript was prepared by SC. SC and ANC edited and reviewed the article. The review and editing process was completed by SC, SS, and ANC, with the approval of all authors.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
Dr. Subhash Chandra is on the Editorial Advisory Board of the Journal of The Open COVID Journal.
ACKNOWLEDGEMENTS
Declared none.


